Key Concepts
Any of the group of organic compounds consisting of carbon, hydrogen, and oxygen that includes sugars, starches, and cellulose. Carbohydrate compounds consist of carbon, hydrogen, and oxygen, with the general chemical formula Cm(H2O)n [Fig. 1]; the hydrogen and oxygen are in a 2:1 ratio. As their name suggests, carbohydrates are hydrates (water-containing solid compounds) of carbon. In the forms of sugars, starches, and cellulose, carbohydrates play a vital role in the lives of plants and animals, both as structural elements and in the maintenance of functional activity. Approximately 50% of the total caloric intake in a typical human diet comes from carbohydrates. See also: Carbon; Cellulose; Hydrogen; Oxygen; Starch; Sugars (sweeteners)
Carbohydrates can be divided into three categories: monosaccharides, oligosaccharides, and polysaccharides. Monosaccharides constitute the simplest form of carbohydrates and cannot be hydrolyzed to smaller units. Oligosaccharides are short chains of 2–10 monosaccharides joined by covalent bonds. Oligosaccharides are often associated with proteins as glycoproteins, or with lipids as glycolipids. Disaccharides account for the most abundant oligosaccharide form. Polysaccharides are long chains of monosaccharides, ranging from 100 to many thousands in length. The most abundant polysaccharide forms are glycogen in animals and starch and cellulose in plants. See also: Glycoprotein; Lipid; Oligosaccharide; Polysaccharide
Monosaccharides
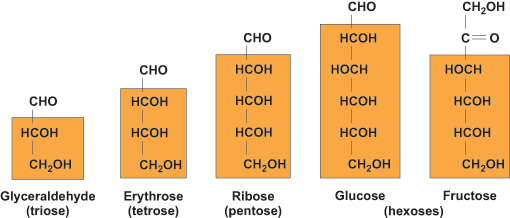
Monosaccharides are classified according to their carbonyl group (a functional group in which a carbon atom is doubly bonded to an oxygen atom) and the number of carbon (C) atoms. Based on the length of the C chain, monosaccharides are referred to as trioses (3C), tetroses (4C), pentoses (5C), and hexoses (6C). Monosaccharides are either aldoses or ketoses, depending on the chemical structure of the carbonyl C as either an aldehyde or a ketone, respectively. Figure 1 presents monosaccharides ranging from 3C to 6C in length, including the two common hexoses, glucose and fructose. Glucose is an aldohexose; its carbonyl C is an aldehyde. Fructose is a ketohexose; its carbonyl C is a ketone. The third common dietary monosaccharide is galactose, which is an aldohexose, like glucose, but differs in the orientation of one C. Overall, metabolically, glucose is the most abundant monosaccharide and the most frequent unit of polysaccharides, including glycogen, starch, and cellulose. Galactose is found in lactose in milk, and fructose is found in sucrose (table sugar) and also as a monosaccharide in fruit and honey. In addition, a significant dietary source of fructose is high-fructose corn syrup. See also: Glucose
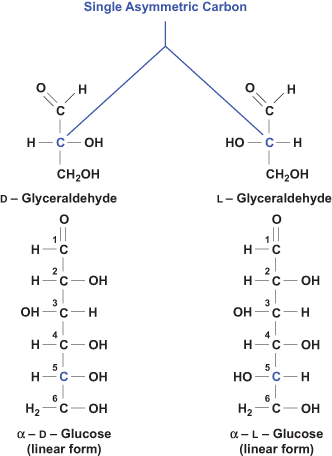
Monosaccharides have specific chemical properties that underlie important biochemical and nutritional traits of carbohydrates. Monosaccharides exist as stereoisomers, meaning that they contain chiral (asymmetric) carbons. A tetrahedral C is chiral if it has four different substituent groups. Chiral compounds have the same chemical composition, but exist as two nonsuperimposable mirror images of each other, and are optically active. These stereoisomers have the same chemical and physical properties, but they rotate plane-polarized light in opposite directions. Optical activity is attributed to the presence of one or more asymmetric or chiral carbons in a monosaccharide. Based on the direction in which the plane-polarized light is rotated, the stereoisomers are designated as d or l in orientation: if light is rotated to the right, the stereoisomer is dextrorotary (d); if to the left, it is levorotary (l). The determining asymmetric C in glucose is C5, and the two stereoisomers of glucose are d- and l-glucose, as shown in Figure 2. Note that the asymmetric C farthest from the carbonyl C determines the d or l configuration. The predominant form of glucose in the diet and in the human body is d-glucose. See also: Optical activity; Stereochemistry
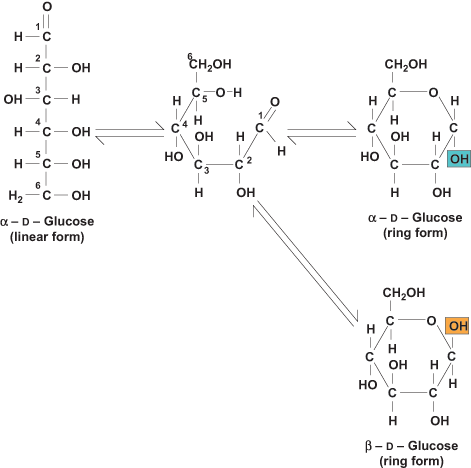
The linear forms of monosaccharides are depicted in Figures 1 and 2; however, monosaccharides commonly exist as ring structures. There is an intramolecular reaction between the carbonyl C and the alcohol ( OH) C to form a carbon ring (Fig. 3). Glucose and galactose are six-membered rings called pyranoses, and fructose is a five-membered ring, or furanose. The formation of ring structures generates a new asymmetric C and two additional anomers of monosaccharides, designated α and β. These are diastereomers, meaning that the α and β forms have different physical and chemical properties (for example, melting point and chemical reactivity). When the anomeric OH group is directed downward, it is α; when it is directed upward, it is β. In general, the ring structure for glucose exists in equilibrium with the more reactive acyclic (or linear) form; however, at physiological pH, the cyclic ring form predominates. See also: pH
OH) C to form a carbon ring (Fig. 3). Glucose and galactose are six-membered rings called pyranoses, and fructose is a five-membered ring, or furanose. The formation of ring structures generates a new asymmetric C and two additional anomers of monosaccharides, designated α and β. These are diastereomers, meaning that the α and β forms have different physical and chemical properties (for example, melting point and chemical reactivity). When the anomeric OH group is directed downward, it is α; when it is directed upward, it is β. In general, the ring structure for glucose exists in equilibrium with the more reactive acyclic (or linear) form; however, at physiological pH, the cyclic ring form predominates. See also: pH
Oligosaccharides
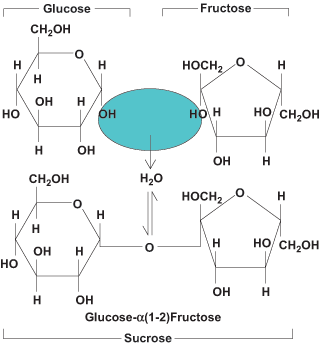
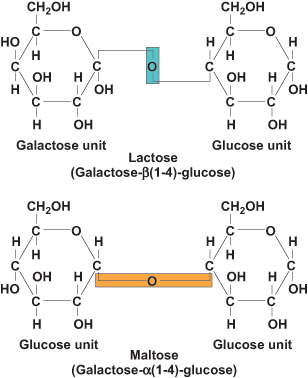
Building on the basic unit of carbohydrates, oligosaccharides (condensation products of 2–10 monosaccharides) constitute the next level of complexity, of which disaccharides are the predominant form. Disaccharides are made up of two monosaccharide units linked by a glycosidic bond. Glycosidic bonds form between the 1C of the upstream unit and the 4C of the downstream unit, generating a 1,4-glycosidic bond. This bond can be in either α- or β-configuration, depending on the orientation of the upstream C1 of the disaccharide pair. Sucrose is unique in that the bond is between the C1 of glucose and the C2 of fructose, generating a 1,2-glycosidic bond (Fig. 4). The most common disaccharides are sucrose [glucose + fructose, α(1,2)], lactose [galactose + glucose, β(1,4)], and maltose [glucose + glucose, α(1,4)] (Fig. 5). Other dietary disaccharides are isomaltose [glucose + glucose, α(1,6)] and cellobiose [glucose + glucose, β(1,4)]. Sucrose is common table sugar, lactose is milk sugar, maltose and isomaltose are breakdown products of starch, and cellobiose is the repeating disaccharide of cellulose. Dietary disaccharides, along with monosaccharides, are referred to as simple carbohydrates. See also: Glycoside
Polysaccharides
Polysaccharides represent the next level of complexity for carbohydrates. Polysaccharide polymers of glucose joined with glycosidic linkages are the most common, and starch, glycogen, and cellulose are the predominant forms. Starch is a major plant polysaccharide and has two forms: amylose and amylopectin. Amylose is linear, and each glucose unit is covalently linked by glycosidic bonds between the 1C in the upstream glucose and the 4C in the downstream glucose, generating an α(1,4)-configuration at each bond. Amylopectin is the branched form, which contains a linear core of glucose units in 1,4-linkage and branch points generated by bonds between C1 of the branching glucose unit and C6 of the linear strand, generating α(1,6)-bonds. Most starches are approximately 70% amylopectin and 30% amylose. Glycogen is the predominant animal polysaccharide and is also branched. Glycogen is the storage form of carbohydrate and is found primarily in the liver and muscle tissue. Cellulose is the major component of the plant cell wall. Cellulose is a linear homoglucose polymer, but the glycosidic bonds between neighboring glucose units are in the β(1,4)-configuration and are not digestible by humans. See also: Glycogen
Metabolism
Overall, the basic chemistry of carbohydrates is metabolically important. Monosaccharides in the d configuration are more prevalent in the diet than l forms and are metabolized in the d configuration. Digestive enzymes are stereospecific. For example, amylase recognizes only the α(1,4)-glycosidic bonds between glucose units in starch. Cellulose, however, is a fiber and cannot be hydrolyzed by amylase because the glycosidic bonds are in the β-configuration. Other digestive enzymes are highly specific for disaccharide digestion; for example, lactase is specific for lactose, and sucrase is specific for sucrose. See also: Carbohydrate metabolism; Enzyme; Nutrition
Glycemic index
The glycemic index is a ranking from 0 to 100 of carbohydrate-containing foods based on how much they increase blood glucose (sugar) levels after eating, compared to glucose (the reference standard, glycemic index = 100). Foods with carbohydrates that break down quickly during digestion have the highest glycemic indexes. Foods ranked 55 or less are considered low glycemic index, 55 to 69 medium glycemic index, and 70 or higher high glycemic index. Low glycemic index foods include green vegetables, chickpeas, and apples. Medium glycemic index foods include ripe bananas, sweet corn, and oatmeal. High glycemic foods include white rice, white potatoes, and watermelon. The glycemic index is an important tool that people with diabetes use for making food choices. See also: Diabetes; Digestion; Food





