If you think converting seawater to fuels that can power ships is a fantasy, think again. Researchers at the U.S. Naval Research Laboratory in Washington, D.C., have, in fact, accomplished this feat. In addition, the U.S. Navy has received a patent for an electrochemical device that removes carbon dioxide (CO2) and hydrogen (H2) gases from seawater, which together can be converted through a catalytic reaction to liquid hydrocarbon fuels that are similar to fossil fuels. Breakthrough technology aside, don’t expect to see U.S. Naval ships powered by fuels from seawater cruising the high seas anytime soon. Making significant quantities of this fuel requires processing a great deal of seawater, which, in turn, requires a large amount of electricity to drive the electrochemical process. Nevertheless, researchers expect efficiency improvements over the next 10 years to bring the concept closer to reality. See also: Carbon dioxide; Catalysis and catalysts; Electrochemical process; Fossil fuel; Hydrogen; Seawater
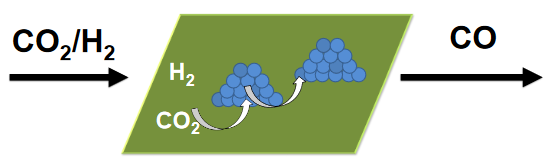
Converting CO2 to hydrocarbons requires two reactions. First, CO2 is converted to CO via the reverse water–gas shift reaction; for example, CO2 + H2 + catalyst1 → CO + H2O. Next, using a second catalyst, the CO produced can be hydrogenated via the Fischer-Tropsch process to hydrocarbons; for example, CO + (2n + 1)H2 + catalyst2 → CnH2n+2 + H2O. See also: Fischer-Tropsch process; Synthetic fuel
Catalysts used in the reverse water–gas shift reaction are often composed of expensive precious metals, such as platinum, and catalyst life is often short. As an improvement, researchers from the University of Rochester, in New York, USA, working in conjunction with Naval researchers, have developed a modified molybdenum carbide catalyst for the reverse water–gas shift reaction made from inexpensive components that appears to be long-lived. Reporting in the journal Energy & Environmental Science (July 2020), these researchers demonstrated that adding potassium to a molybdenum carbide catalyst improved the efficiency of converting CO2 into CO and that the catalyst remained active throughout a 10-day study. Whether or not ships will ever be efficiently powered by fuels from seawater is yet to be determined. It is conceivable, however, that in the future the process can be improved enough to cost-effectively produce a sufficient amount of jet fuel to power jets aboard an aircraft carrier. See also: Aircraft fuel; Molybdenum; Molybdenum alloys; Potassium





