Defying long-held expectations, chemists from Colorado State University created a polyester biopolymer that can be decomposed back into its monomer constituents. Their accomplishment was all the more surprising because the material with which they started, the cyclic ester (lactone) gamma-butyrolactone, was considered too thermodynamically stable to be a promising candidate for polymerization reactions under practical conditions. They announced their work in the journal Nature Chemistry (November 2015). See also: Biopolymer; Lactone; Polyester resins; Polymer
Polyethylene and other thermoplastic polymers are generally not fully recyclable in a chemical sense: They can be melted and formed into new products, but they cannot be converted back into their original starting materials. Similarly, biopolymer plastics made from renewable resources are not truly recyclable because even if they can degrade into environmentally safe compounds, those compounds are chemically different from the monomers used to build the biopolymers. For example, the biopolymer poly(γ-butyrolactone) degrades to γ-hydroxybutyric acid, a naturally occurring metabolite in the body, rather than to γ-butyrolactone. See also: Polymers from renewable resources; Polymer recycling and degradation; Polyolefin resins
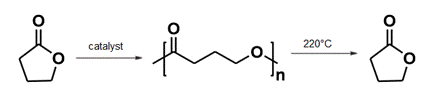
However, the Colorado State researchers successfully took biologically derived γ-butyrolactone and, through a ring-open polymerization reaction, turned it into both linear and cyclic polymers. Then by heating the linear poly(γ-butyrolactone) for one hour at 220°C and the cyclic poly(γ-butyrolactone) for one hour at 300°C under a nitrogen atmosphere, they depolymerized the compounds back into the monomer. Notwithstanding whatever applications are found for their work, it represents a remarkable achievement in polymer science. See also: Ring-opening polymerization





