Mercury and its compounds are highly toxic—that is, they cause harmful effects to living organisms. Mercury pollution is found in water, air, and soil worldwide. According to the United Nations Environment Programme, the top anthropogenic sources of mercury pollution are small-scale gold mining (37.7%), coal combustion (21%), nonferrous metal production (15%), and cement production (11%). Mercury that is emitted into the atmosphere may eventually enter surface water and groundwater, where microorganisms convert the element to methylmercury—a persistent, bioaccumulative, and toxic pollutant (PBT) that builds up in the food web. Human exposure to methylmercury most often occurs when people eat fish or shellfish containing high levels of methylmercury. Methylmercury poisoning in humans can cause severe illness, including central nervous system and digestive-system damage as well as kidney failure, and even death. See also: Air pollution; Cement; Food web; Mercury (element); Persistent, bioaccumulative, and toxic pollutants; Poison; Toxicology; Water pollution

Researchers from Flinders University, Australia, reported in the journal Green Chemistry (May 2022) the discovery of a polymer—a sulfur-limonene polysulfide—made from industrial waste materials that bonds to, and rapidly extracts, mercury pollution from water. The researchers obtained the d-limonene (a terpene) as a waste byproduct from the peels of oranges and other citrus fruits. They obtained elemental sulfur, consisting mainly of eight-membered sulfur rings, as a waste byproduct of petroleum refining. At a temperature of 180 degrees Celsius, the polymerization of sulfur with d-limonene occurs, producing the polysulfide. An additional benefit of using the sulfur-limonene polysulfide is that this substance changes color from dark red to yellow on bonding to mercury—a chemical reaction that can be used to indicate its effectiveness. See also: Polymerization; Sulfur; Terpene
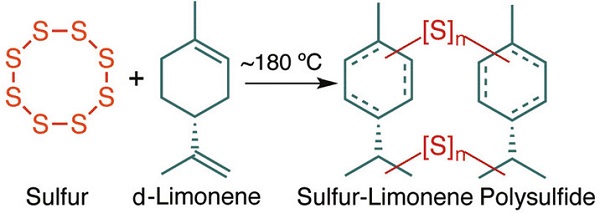
To absorb mercury, the researchers applied limonene polysulfide as a thin coating on a silica substrate. In laboratory testing, the polysulfide removed up to 99 percent of all mercury from water samples in only a few minutes. Mathematical modeling followed laboratory testing to simulate how the polysulfide could be used in real-world applications. Based on experimental and modeling results, the researchers envision using limonene polysulfide in environmental remediation applications to remove mercury from groundwater. In addition, they are exploring its use in water filtration to produce safe drinking water. See also: Model theory; Simulation; Water treatment





