Key Concepts
The temperature at which the liquid and vapor phases are in equilibrium with each other at a specified pressure. At the boiling point, the transition from the liquid to the gaseous phase occurs in a pure substance. Therefore, the boiling point is the temperature at which the vapor pressure of the liquid is equal to the applied pressure on the liquid. The boiling point at a pressure of 1 atmosphere is called the normal boiling point (see illustration).
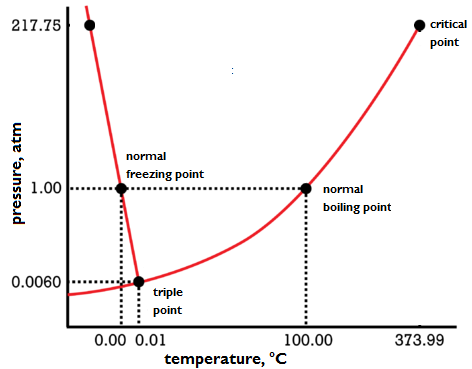
For a pure substance at a particular pressure P, the stable phase is the vapor phase at temperatures immediately above the boiling point and is the liquid phase at temperatures immediately below the boiling point (see illustration). The liquid-vapor equilibrium line on the phase diagram of a pure substance gives the boiling point as a function of pressure. Alternatively, this line gives the vapor pressure of the liquid as a function of temperature. The vapor pressure of water is 1 atm (101.325 kilopascals) at 100°C, the normal boiling point of water. The vapor pressure of water is 3.2 kPa (0.031 atm) at 25°C, so the boiling point of water at 3.2 kPa is 25°C. The liquid-vapor equilibrium line on the phase diagram of a pure substance begins at the triple point (where solid, liquid, and vapor coexist in equilibrium) and ends at the critical point, where the densities of the liquid and vapor phases have become equal. For pressures below the triple-point pressure or above the critical-point pressure, the boiling point is meaningless. Carbon dioxide has a triple-point pressure of 5.11 atm (518 kPa), so carbon dioxide has no normal boiling point. See also: Triple point; Vapor pressure
The normal boiling point is high for liquids with strong intermolecular attractions and low for liquids with weak intermolecular attractions. Helium has the lowest normal boiling point, 4.2 kelvin (−268.9°C). Some other normal boiling points are 111.1 K (−162°C) for methane (CH4), 450°C for triacontane (n-C30H62), 1465°C for sodium chloride (NaCl), and 5555°C for tungsten (W).
The rate of change of the boiling-point absolute temperature Tb of a pure substance with pressure is given by the equation below.
ΔHvap,m is the molar enthalpy (heat) of vaporization, and ΔVvap,m is the molar volume change on vaporization.
The quantity ΔHvap,m/Tb is ΔSvap,m, the molar entropy of vaporization. The molar entropy of vaporization at the normal boiling point (nbp) is given approximately by Trouton's rule: ΔSvap,m,nbp ≈ 87 J/mol K (21 cal/mol K). Trouton's rule fails for highly polar liquids (especially hydrogen-bonded liquids). It also fails for liquids boiling at very low or very high temperatures, because the molar volume of the vapor changes with temperature and the entropy of a gas depends on its volume.
When a pure liquid is boiled at fixed pressure, the temperature remains constant until all the liquid has vaporized. When a solution is boiled at fixed pressure, the composition of the vapor usually differs from that of the liquid, and the change in liquid composition during boiling changes the boiling point. Thus the boiling process occurs over a range of temperatures for a solution. An exception is an azeotrope, which is a solution that boils entirely at a constant temperature because the vapor in equilibrium with the solution has the same composition as the solution. In fractional distillation, the variation of boiling point with composition is used to separate liquid mixtures into their components. See also: Azeotropic mixture; Distillation; Phase equilibrium





