Sunscreens are polluting the ocean, and increasing evidence points toward some ultraviolet-light–absorbing chemicals found in sunscreens as being harmful to marine life—particularly corals at popular tourist destinations. While working toward finding environmentally friendlier sunscreens, researchers have found that certain marine organisms produce protective UV-absorbing chemicals, called mycosporine-like amino acids (MAAs). MAAs are colorless, water-soluble compounds with relatively low molecular weight and are found in cyanobacteria, marine algae, and corals. They absorb UV light strongly from 310 to 365 nanometers (nm). One such source of MAAs is the red algae, Porphyra umbilicalis, commonly known as nori, the edible sushi wrap. See also: Algae; Amino acid; Cyanobacteria; Reef; Water pollution

Even though corals occupy less than one percent of the ocean floor, they provide a habitat for 25 percent of all ocean fish species. Increasingly, coral reefs are experiencing environmental stress caused by higher-than-normal water temperature, inducing the colorful algae (zooxanthellae) that live within the coral tissue to produce toxins, resulting in corals expelling the zooxanthellae and appearing white. This is known as coral bleaching. Zooxanthellae provide oxygen and energy to corals and remove their waste. In their absence, corals are more susceptible to disease and, potentially, death. See also: Coral bleaching; Coral diseases; Coral reef complexity; Marine ecology; Ocean warming; Severe coral bleaching endangers Great Barrier Reef
Several UV absorbers in sunscreens, such as oxybenzone, octinoxate, and 4-methylbenzylidene camphor, have also been shown to cause coral bleaching, according to a report in the Archives of Environmental Contamination and Toxicology (February 2016). In addition to bleaching, oxybenzone caused DNA damage, endocrine disruption, and skeletal deformities in developing coral. Of the coral-toxic UV absorbers, oxybenzone is the most widely used globally (found in more than 3500 sunscreen products) because it absorbs ultraviolet light in both UVA (400 to 320 nm) and UVB (320 to 290 nm) ranges. Generally, the organic UV absorbers found in sunscreens have higher absorptivity in the UVB range. Both UVA and UVB rays can lead to skin cancer, but the greatest sun exposure comes from UVA rays, which are present all day long and can penetrate clouds and glass. See also: Ultraviolet radiation (biology)
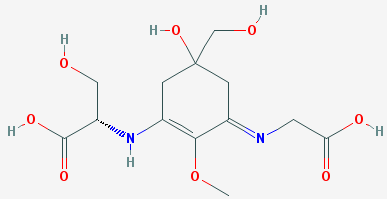
Shinorine (shown below) is one of more than 20 MAAs that have been identified to show strong UV protection. However, on a dry weight basis, MMAs make up only about one percent of Porphyra, making extraction of a sufficient supply of shinorine a less-than-optimal process for producing commercial sunscreens, although it is done. To overcome the supply problem, researchers reported in ACS Synthetic Biology (January 2018) that they had genetically engineered cyanobacteria to photosynthesize shinorine. Although further work is required to scale-up shinorine production, regulatory approval for this environmentally friendly and biosynthesized UV absorber and similar compounds is not expected to be a problem. See also: Genetic engineering