Key Concepts
A class of chemicals characterized by their common chemical structure and potentially fatal nervous system effects.
Development and historical use
Research into organophosphate insecticides led to the development of nerve agents in the 1930s. Nerve agents are known by their common names and by a one- to two-letter military designation. The first generation are known as the “G” agents: tabun (GA; Fig. 1), soman (GD), sarin (GB), and cyclosarin (GF). G agents were used in the Iran-Iraq war during the 1980s, and in attacks against civilians in Iraq and Syria. Use of these chemicals is considered an act of terrorism and a violation of the Chemical Weapons Convention. As an example, the Aum Shinrikyo cult used GB in the Tokyo subway attacks in 1995, in which 12 people died and more than 1000 were injured after exposure. GB was also used in an attack in 1994 in Matsumoto, Japan, that killed 7 people and injured more than 600. The subsequent “V” series of nerve agents (such as VX) are even more lethal than the G agents and are more persistent in the environment. Aum Shinrikyo also exposed people to VX in Japan in 1994 and 1995, and VX was used in the 2017 assassination of Kim Jong-nam of North Korea in Malaysia. See also: Bioterrorism; Insecticide; Organophosphorus compound
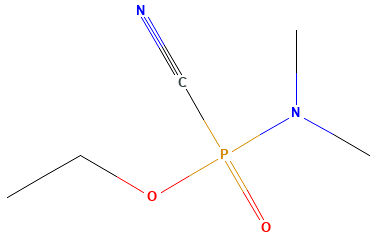
Further enhancements of the manufacturing, transportation, and storage of nerve agents resulted in the development of a two-part delivery system that activates the chemicals only when mixed. These are known as “binary” nerve agents. Fourth-generation agents, or FGA, were developed that have higher persistence and at least equivalent toxicity to the V-series agents, such as those allegedly used in the United Kingdom in 2018. See also: Toxicology
Biological effects
Nerve agents are liquids at room temperature and are delivered as a liquid aerosol (small liquid particles suspended in air), as a vapor, or by direct contact, depending on the agent used. Absorption occurs through inhaling aerosol droplets or vapor, by swallowing the agent, or from direct skin exposure. See also: Aerosol
Nerve agents prevent the acetylcholinesterase enzyme (AChE) in cholinergic neuronal junctions from functioning properly. Proper functioning is needed for balanced regulation of the nervous system. As a result, acetylcholine (a neurotransmitter) builds up, causing nervous system dysfunction throughout the body through continued activation of the cholinergic receptors in the neuronal junction. The two main types of acetylcholine receptors are the nicotinic and muscarinic receptors. Each type is involved in different aspects of the nervous system, from controlling secretion of sweat glands on the skin to transmitting electrical impulses of neurons in the brain. Nerve agents initially bind to AChE in a reversible process, causing enzyme inhibition and immediate acetylcholine buildup. The agents permanently inactivate AChE within minutes to hours, depending on the particular type of agent, in a process called “aging.” The body can take weeks to regenerate the enzyme to baseline levels. See also: Acetylcholine; Nervous system (vertebrate); Neurobiology; Poison; Synaptic transmission
Nervous system toxicity causes a well-described group of clinical signs and symptoms, or toxidrome. Nerve agent exposures generally lead to clinical signs and symptoms referred to as the cholinergic toxidrome. The mnemonic DUMBBBELSS describes the classic cholinergic muscarinic signs and symptoms:
- diarrhea (excessive)
- urination
- miosis (small pupils)
- bronchospasm (constriction of airways in the lungs)
- bradycardia (decreased heart rate)
- bronchorrhea (lung secretions)
- emesis (vomiting)
- lacrimation (tearing)
- salivation
- sweating
A second mnemonic (MTWThF) describes a further subset of cholinergic nicotinic signs and symptoms:
- mydriasis (large pupils)
- tachycardia (elevated heart rate)
- weakness (of muscles, including respiratory muscles)
- twitching (muscle contraction and relaxation)
- fasciculations (muscle tremors)
As a result of direct effects on the central nervous system, patients also might exhibit altered mental status, seizures, coma, or respiratory depression. See also: Central nervous system; Respiratory system
Because of their high potency, nerve agents can be fatal even when a patient is only exposed to small amounts (1–10 milliliters). Depending on the agent and route of exposure, death can occur within minutes. Most deaths occur soon after exposure due to central nervous system respiratory effects. Complications can include coma, prolonged respiratory failure, and prolonged neurological complications.
Detection
Nerve agents can be detected directly in the environment or through biomarkers in exposed persons. However, nerve agents can quickly cause death, so medical treatment for persons suspected of being exposed should not be delayed while waiting for test results to confirm exposure. Environmental detection technologies consist of rapid on-scene test kits and equipment that can detect the presence of nerve agents. Handheld test kits involve a color-changing chemical reaction that occurs within minutes to detect nerve agents. More sophisticated surveillance monitors sample the air in near-real time and use a variety of spectroscopy technologies to detect nerve agents. See also: Biomarkers: key to exposure reconstruction; Biosensor; Spectroscopy
A patient’s exposure to nerve agents can be confirmed indirectly through measurement of biomarkers in blood samples. Exposure is typically confirmed by decreased levels of cholinesterase activity measured against a reference range or a pre-exposure baseline in the same patient. Exposure can also be directly confirmed by measuring nerve-agent metabolites in the urine, although this more specialized testing method is not routinely available. For indirect testing methods, two types of cholinesterase enzymes can be used to assess for nerve agent exposure:
- Butyrylcholinesterase (BuChE), which circulates in the plasma, will decrease rapidly after exposure, and can recover within days after mild exposure.
- Erythrocyte AChE, which is attached to red blood cells, will decrease more slowly after exposure, and can take weeks to recover.
These indirect specialized tests can be difficult to interpret because of several factors, including not knowing a pre-exposure baseline, uncertainty about the timing of blood sample collection in relation to the exposure, and the results being affected by unrelated illnesses or disease states.
Clinical management
Since their development, nerve agents have been recognized for their significant toxicity and have repeatedly demonstrated their potential to cause death. Effective clinical response requires early recognition of nerve agent exposure and addressing the priorities of clinical management: decontamination, supportive care, and medical therapy.
Decontamination
Decontamination is an important step required in clinical management. A nerve agent that has saturated clothing or skin can continue to be absorbed by patients and can secondarily contaminate healthcare workers or bystanders. Careful decontamination by providers wearing appropriate personal protective equipment (Fig. 2) must be performed as soon as possible after exposure, ideally before leaving the scene where contamination occurred (Fig. 3). Contaminated clothing is removed from patients, who are then washed with soap and water or reactive skin decontamination lotion, if available. Decontamination should be performed expeditiously to ensure minimal delay in safe administration of an antidote. See also: Hazardous waste


Supportive care
Supportive care that is focused on the patient’s airway, breathing, and circulation is often required. Because of excessive secretions, patients might require endotracheal intubation (insertion of a breathing tube) and mechanical ventilation (use of a machine to control breathing) to secure a patent airway. These interventions also might be required to prevent respiratory compromise due to paralysis of the respiratory muscles or decreased respiratory drive from the central nervous system. As a result of fluid losses from secretions and decreases in blood pressure or heart rate, patients also might require IV fluids or medications to maintain safe hemodynamics.
Medical therapy
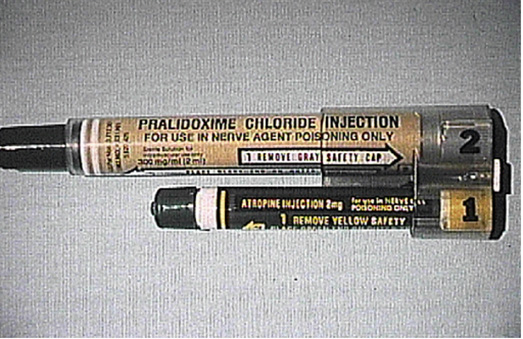
Medical therapy administered as soon as possible after exposure can be critical in cases of suspected nerve agent exposure. Medical therapy can include specific antidotes against nerve agents and medications to treat seizures. Two specific antidotes widely used in combination for nerve agent exposure are (1) atropine, which helps prevent excess acetylcholine from causing cholinergic muscarinic signs and symptoms, and (2) an oxime, such as pralidoxime, which directly acts on the nerve agent to remove it from the acetylcholinesterase enzyme, to prevent “aging.” See also: Atropine
Both antidotes can be readily administered from auto-injector kits (Fig. 4), which are spring-loaded syringes that contain a set medication dose and can be administered by nonmedical personnel. Atropine should be given as soon as possible after exposure; it can immediately prevent or reverse dangerous effects of very high levels of acetylcholine excess, even if AChE has “aged.” Pralidoxime also must be administered soon after exposure. It will not be effective if “aging” has occurred because it cannot reverse this permanent inactivation of AChE. Benzodiazepines are another medication used to treat central nervous system–mediated seizures or altered mental status caused by nerve agents. Benzodiazepines also can be given by auto-injector. In severe cases, all treatments might have to be given continuously for days until symptoms improve or cholinesterase testing shows a return to baseline.
Recovery
Recovery is marked by improvement of symptoms and indirectly by AChE function. How long symptoms last varies based on the type of exposure agent and the length and quantity of exposure. For example, persons exposed to GB in Japan continued to have symptoms three weeks after exposure. Those symptoms included fatigue, dysesthesia (abnormal sensations), and eye pain. All symptoms had cleared by three months after exposure. Additionally, symptoms such as lack of muscular coordination (ataxia), neuropathy, and posttraumatic stress disorder have been reported, with effects lasting days to months after an exposure. See also: Posttraumatic stress disorder
[Disclaimer: The findings and conclusions of this overview are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. We have no financial disclosures.]





