Key Concepts
A list of chemical elements arranged along horizontal rows in increasing atomic number. It is organized such that the vertical columns consist of elements with remarkably similar properties (see illustration). The first column, known as the alkali metals (albeit with hydrogen, a nonmetal on top), contains elements with just one outer (valence) electron. The last column has completely filled valence orbitals leading to chemically inert elements called the noble gases. The position of elements in the periodic table provides a powerful method of classifying not only the physical properties of elements but also their expected properties in molecules and solids. See also: Alkali metals; Atomic number; Electron configuration; Noble gases; Valence
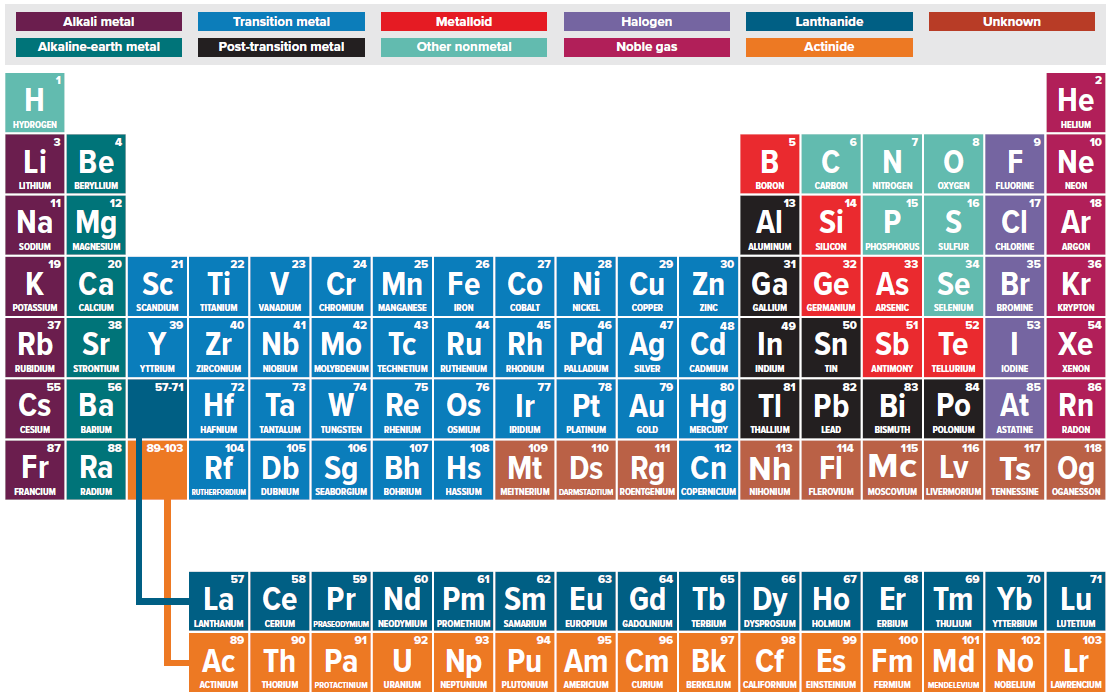
The periodic table dates back to around 1870 when the Russian chemist D. Mendeleev used the similarities in chemical reactivity attributed to different elements to group them according to increasing atomic mass. However, this left blank spaces that Mendeleev boldly predicted would be filled by elements that were then undiscovered. Not only did other scientists discover missing elements including scandium, gallium, and germanium, but remarkably these elements possessed properties similar to Mendeleev's predictions. Although the layout of the periodic table has changed over time with the addition of new elements, the essential information remains comparable to the original periodic table. See also: Atomic mass
Groups
The modern periodic table is divided into 18 columns called groups or families (see illustration). Elements in each family tend to have similar properties. In column 1, each alkali metal is soft, relatively low-melting, and highly reactive toward air and water. Column 2 contains the alkaline earth metals, which have higher melting points and are less reactive. Columns 3–12 are filled by the transition metals, which are shiny and good conductors of both heat and electricity. Columns 13–18 are often discussed along with columns 1 and 2, and collectively they are known as the main group or representative elements. Column 15, headed by nitrogen, is known as the pnictogens; column 16, beginning with oxygen, as the chalcogens; column 17, starting with fluorine, as the halogens; and column 18, starting with helium, as the noble gases. See also: Alkaline-earth metals; Transition elements
Periods
The horizontal rows of the periodic table are called periods. Atomic mass generally increases from left to right across a period, while atomic size generally decreases. The decrease in size is due to incomplete screening of the positive nuclear charge by the valence electrons, which causes the outer electron shells to contract. Other properties follow periodic trends, including the ionization potential (the energy needed to remove an electron), electron affinity (the energy released on accepting an electron), and electronegativity (the ability of an atom in a compound to attract electron density). See also: Atomic structure and spectra; Electron affinity; Electronegativity; Ionization potential
After element 57 (lanthanum) comes a series of 14 metallic elements numbered 58–71 with very closely related properties. These originally were named the rare earths since they are all nearly the same size, have similar chemical reactivity, and are difficult to separate. However, they are not rare and are now more appropriately called the lanthanides. Technically the lanthanides should be placed between elements 57 (lanthanum) and 72 (hafnium). Since this would nearly double the width of the periodic table, they are usually placed below all the other elements. Keeping in mind that size decreases left to right across a period, hafnium (72) is much smaller than its neighbor lanthanum (57). In fact, hafnium is essentially the same size as the element above it, zirconium (40). With comparable chemical reactivity and size, zirconium and hafnium are difficult to separate. This also suggests that the second and third rows of the transition metals will possess many common chemical features, as they do. The decrease in size due to the 14 elements between lanthanum and hafnium is called the lanthanide contraction. Below the lanthanides are 14 more metallic elements (90–103) called the actinides. See also: Actinide elements; Lanthanide contraction; Rare-earth elements
Elements
Each box in the periodic table contains a one- or two-letter symbol representing a different element such as C for carbon (6) or Sg for Seaborgium (106) [see illustration]. The number in the upper left corner is the atomic number indicating how many protons are in the atom's nucleus. The atomic mass generally appears below the symbol indicating the average mass observed for that element. For example, most carbon (99%) contains 6 protons and 6 neutrons, leading to a mass of 12. However, since about 1% of carbon has an extra neutron, the average mass of carbon as given in the periodic table is 12.011. If an element has no stable isotopes, then the mass of the longest-lived isotope is given in parentheses. More complex periodic tables often include information on density, melting points, and boiling points. Separate tables are available, indicating crystal structures, magnetic properties, radioactive decay patterns, and other properties.
All of the elements in the periodic table have been officially ratified by the International Union of Pure and Applied Chemistry (IUPAC). When a newly discovered element has been independently verified, the original discoverer (often a team) earns the right to propose a name to IUPAC. The elements beyond 92 (uranium) do not occur naturally and are produced using nuclear reactions. Elements beyond 100 are not particularly useful, since they generally undergo rapid nuclear decay by emitting radiation. See also: Transuranium elements
Other properties
Many periodic tables include a stair-step line separating metals from the metalloids and nonmetals. Most elements are metals and generally have physical properties that include luster (high reflectivity), good conductivity for both electricity and heat, high density, usually high melting points, ductility (the ability to be drawn into a wire), and malleability (the ability to be hammered into thin sheets). The chemical properties of most metals include corrosivity such as iron rusting and silver tarnishing, as well as the ability to give up electrons. Nonmetals are found to the right of the metals and their characteristics are the inverse. This means most have no luster (appearing dull), are poor conductors of electricity and heat, have low density, low melting points, and are brittle. Chemically, nonmetals like to gain electrons and often react with metals to produce salts. For example, combining an alkali metal with one valence electron with a halogen that needs one electron to complete its valence shell produces an alkali halide salt such as sodium chloride or common table salt. Metalloids straddle the stair-step line and often have properties in between metals and nonmetals. With intermediate conductivities, elements such as silicon form important semiconductors used in computer chips and solar cells. See also: Metal; Metalloid; Nonmetal; Semiconductor





