The field of chemistry that includes all chemical measurements, such as the measurements of atomic and molecular weights and sizes, gas volumes, vapor densities, deviation from the gas laws, and the structure of molecules. In the long struggle to determine the relative weights of the atoms, scientists relied upon combining ratios, specific heats, and measurements of gas volumes. All such measurements, and the calculations that relate them to each other, constitute the field of stoichiometry. Since measurements are expressed in mathematical terms, stoichiometry can be considered to be the mathematics of general chemistry. Thus, stoichiometry is not part of inorganic, organic, physical, or analytical chemistry, but is an essential part of all of them. Chemistry is an exact science, and it depends upon exact measurements of weights, lengths, and volumes, and on the amounts of energy which are absorbed or evolved in chemical reactions.
In a more usual usage, the term stoichiometry refers to the relationships between the measured quantities of substances or of energy involved in a chemical reaction; the calculations of these quantities include the assumption of the validity of the laws of definite proportions and of conservation of matter and energy. See also: Conservation of energy; Conservation of mass
Laboratory measurements are made in terms of a unit of weight (grams, ounces, and such), volume (milliliters and such) or energy (ergs, calories, and such), but since atoms and molecules of different substances have different weights, it is necessary to convert numbers of atoms or molecules into the measured quantities. These quantities can be expressed in any convenient units. In laboratory work, weights are usually expressed in grams, but in chemical engineering, in pounds or tons. See also: Atomic mass; Gram-molecular weight; Molecular weight
A typical stoichiometric problem involves predicting the weight of reactant needed to produce a desired amount of a product in a chemical reaction. For example, phosphorus can be extracted from calcium phosphate, Ca3(PO4)2, by a certain process with a 90% yield (some calcium phosphate fails to react or some phosphorus is lost). In a specific problem, it might be necessary to determine the mass of calcium phosphate required to prepare 16.12 lb of phosphorus by this process. The balanced equation for the preparation is shown in reaction (1).
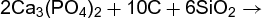
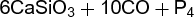 (1)
(1)
In this reaction, 2 moles of calcium phosphate are required to produce 1 mole of phosphorus. Two moles of calcium phosphate have a mass of 620 lb, and 1 mole of phosphorus as P4 has a mass of 124 lb. Using these relationships, calculation (2)
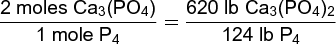
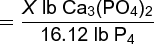 (2)
(2)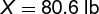
is made. Since the yield of phosphorus is only 90%, extra Ca3(PO4)2 must be used: 88.1 lb is the mass of calcium phosphate required to yield 16.12 lb of phosphorus by this process.
Calculations of this sort are important in chemical engineering processes, in which amounts and yields of products must be known. The same reasoning is used in calculations of energy generated or required. In this case, the energy involved in the reaction of a known weight of the material in question must be known or determined. There are many variations on this theme. For example, if it is necessary to determine the composition of a mixture of copper and zinc powders, a given weight of the material can be dissolved in hydrochloric acid, which reacts with the zinc, but not with the copper [reaction (3)].
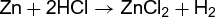 (3)
(3)
From the volume of hydrogen gas liberated, the amount of zinc dissolved can be calculated, and hence the composition of the mixture. Again, if 5.0 g of sodium is placed in a vessel containing 5.0 g of chlorine, a violent reaction takes place [reaction (4)].
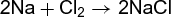 (4)
(4)
However, some of the sodium remains unattacked. It can be calculated that 5.0 g of chlorine can react with only 3.2 g of sodium; 1.8 g of sodium will remain. The chlorine is said to be the limiting reagent—when it is used up, the reaction must stop. See also: Avogadro's law; Combining volumes, law of; Dalton's law; Definite composition, law of; Multiple proportions, law of; Specific heat
The calculations discussed in this article involve compounds in which the ratio of atoms is generally simple. For a discussion of compounds in which the relative number of atoms cannot be expressed as ratios of small whole numbers. See also: Nonstoichiometric compounds





