As particulate matter with at least one dimension that is less than 100 nm, nanoparticles are the minuscule building blocks of new commercial products and consumer materials in the emerging field of nanotechnology. Nanoparticles are being discovered and introduced in the marketplace at a very fast pace. Also, commercial interest in nanotechnology has significantly increased, translating into more than a multibillion-dollar investment from public and private sources. Among several unique properties, nanoparticles have an exceptionally large surface area–to-volume ratio, which is the most important of the characteristics that are responsible for their widespread use in an array of industries. Unfortunately, their small size and corresponding high surface area often create a number of problems. For instance, the outer layer of atoms may have a different composition, and therefore a different chemistry, from the rest of the particle. Furthermore, nanoparticle surfaces are sensitive to changes in redox conditions, pH, ionic strength, and the types of microorganisms present. The synthesis of metal nanoparticles has been the subject of intense research, primarily because of their unique properties and their potential applications from a technological point of view. The optical, magnetic, electronic, and catalytic properties of these materials depend on their morphology and size distribution. Noble-metal nanoparticles are of particular interest because of their close-lying conduction and valence bands, in which electrons move freely. These free electrons generate surface plasmon bands dictated by the particles' size, shape, and immediate surroundings. Notably, the fascinating color of the noble-metal nanoparticles depends on both the size and the shape of the particles as well as the refractive index of the encasing medium. See also: Conduction band; Nanoparticles; Nanotechnology; Plasmon; Plasmonics; Refraction of waves; Valence band
The adverse impact of nanomaterials on health and the environment and the risks associated with generating and handling nanomaterials are important challenges. The reagents and chemicals used to make nanoparticles through conventional syntheses are often toxic. These chemicals include reducing agents such as borohydrides or hydrazine derivatives, and capping agents (to prevent further growth) such as polyvinylpyrrolidone. The high chemical activity of engineered nanoparticles with vast surface areas is usually the main reason for the occurrence of undesirable and often irreversible processes such as aggregation, which reduces the surface area and the interfacial free energy, thus diminishing the particles' reactivity. To prevent aggregation, it is very important to increase the stability of the nanoparticles during storage and transportation and throughout their overall life cycle. Most stabilization methods include dispersant molecules such as surfactants or polyelectrolytes. These dispersant molecules not only change the chemistry and physics of the nanoparticle surface, but also generate a tremendous waste stream because they occupy a large (more than 50%) mass fraction of a nanoparticle. To avoid this contamination and the subsequent adverse effects on the environment, it is vital to search for environmentally benign stabilization and functionalization pathways and for biocompatible (that is, nonimmunogenic, nontoxic, and hydrophilic) stabilizing agents. The fate of nanoparticles and their transport is often dictated by their surface functionalization. See also: Nanoparticle risk informatics; Surfactant; Toxicology
Biomimetic approach
Biomimetic strategies, which minimize any associated hazards by using green chemistry approaches such as naturally occurring antioxidants (rather than toxic chemicals), have been developed to prepare nanomaterials. In the synthesis of metal nanoparticles, there are three possible means for embracing green chemistry: (1) the solvent chosen, (2) the reducing agent employed, and (3) the capping agent or dispersing agent used. Ideally, all these avenues need to be exploited in the synthesis of nanoparticles. By learning from nature, a wide variety of nanoparticles have been produced using completely safe and benign materials, such as sucrose, vitamin B1 (Fig. 1), vitamin B2 (riboflavin), vitamin C (ascorbic acid), coffee and tea extracts (Fig. 2), beet juice, and even agricultural waste products (for example, grape pomace), in the complete absence of any additional reducing or capping agents. Research has shown the powerful health benefits of the antioxidants found in tea, wine, and red grape pomace (a major by-product of winemaking), and now these antioxidants can be tapped for cleaner chemistry. See also: Antioxidant; Green chemistry; Nanochemistry; Vitamin
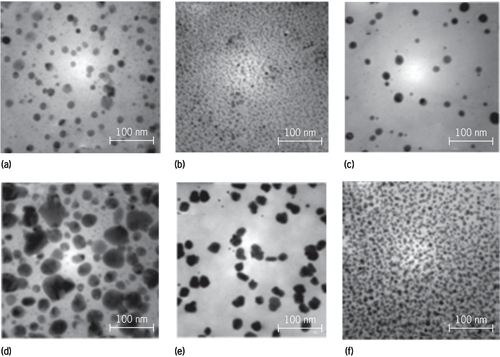
Biomineralization is a natural synthesis process, assisted by biological molecules that nature has developed over a period of millions of years to generate multifunctional nanomaterials such as nanometals and nanosilica. Plants, algae, and bacteria generate metal nanoparticles that are a direct outcome of detoxification routes. For example, bacteria are known to make crystalline magnetic nanoparticles for course plotting, navigation, and coordination. Bacteria have evolved mechanisms to prevent the excessive buildup of toxic metals; these may include enzymatic reduction of the metal ions to less toxic zero-valent metals, thus decreasing membrane permeability and circumventing uptake. Often, the nanomaterials are synthesized under genomic regulation dictated by cellular pathways, culminating in nanoparticles of specific sizes, shapes, and morphologies. The salient feature is that these assembly processes proceed under ambient temperature and pressure conditions, unlike conventional synthesis in the laboratories, in which the use of toxic and noxious reagents and elevated pressures and temperatures is commonplace. These instructions from nature are being adopted for the in vitro synthesis of such materials under relatively benign conditions using peptide, protein, or DNA/RNA biotemplates. Multidisciplinary researchers from biology, chemistry, materials science, and engineering are now mimicking and exploring these pathways to generate synthetic materials using biological methods.
Alternative energy input
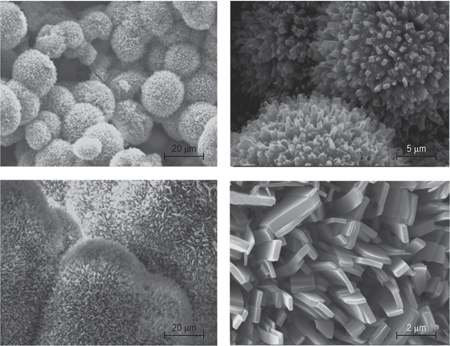
Rapid microwave (MW) heating has been used as a promising new method for the one-pot synthesis of metallic nanostructures in solutions and has been demonstrated in the preparation of silver (Ag), gold (Au), platinum (Pt), and gold-palladium (Au-Pd) nanostructures. This heating strategy allows not only the preparation of spherical nanoparticles within a few minutes, but also the formation of single crystalline polygonal plates, sheets, rods, wires, tubes, and dendrites. The morphologies and sizes of the nanostructures can be controlled by changing various experimental parameters, such as the concentration of metallic-salt precursors, the concentration of surfactant polymers, the chain length of the surfactant polymers, the type of solvents, and the operating reaction temperature. In general, nanostructures with smaller sizes, narrower size distributions, and a higher degree of crystallization have been obtained more often using MW heating than using conventional oil-bath heating. This MW approach is a viable avenue for the greener synthesis of nanomaterials with desirable attributes such as shorter reaction times, reduced energy consumption, and better product yields. See also: Microwave
A simple, green, and expeditious approach has been developed by using beet juice for the fabrication of hybrid AgCl/Ag plasmonic nanoparticles under microwave irradiation, which is an unusual top-down hydrothermal synthesis. In this method, beet juice, which is an abundant sugar-rich agricultural product, served as a reducing reagent; the method uses no additional surfactant or reducing agent. Metal nanoparticles of Au, Ag, Pd, and Pt have been synthesized in water using red grape pomace, which serves as both a reducing and a capping agent. The particles are formed within a few seconds when exposed to microwave irradiation at a power level as low as 50 W.
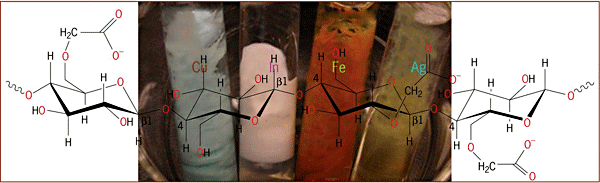
Also, the use of naturally occurring biorenewable polymers, such as cellulose (Fig. 3) and chitosan, has been demonstrated to produce nanocomposites. See also: Cellulose; Cellulose nanocomposites; Polymers from renewable resources
Nanocatalysis
Catalysis is an integral part of several significant industrial processes that play a critical role in the conversion of basic raw materials into useful products. The progress in the synthesis of nanostructured materials has led to their manipulation at the atomic level, and the field of catalysis has been immensely affected by these developments, with the emerging field of nanocatalysts having found close links with traditional catalysts. Among various other applications, it is well known that the catalytic properties of nanomaterials can be altered by changing their size, shape, and composition, which enables the design of highly efficient catalysts with unique selectivity via control of their shapes. The research activity in the field of nanocatalysis has mushroomed, with most of the studies focusing on the higher activity, selectivity, and greater ease of separation from the reaction medium using magnetic nanocatalysts. An external magnet simply separates these catalysts from the reaction mixtures, thus providing a truly sustainable process. Often referred to as quasi-homogeneous systems, nanocatalysts are emerging as sustainable entities that bridge the gap between two classic types of catalysts, namely homogeneous and heterogeneous catalysts, encompassing the beneficial attributes of both the systems. Nanocatalysis, although not quite homogeneous, enables high activities and selectivities that are close to those of homogeneous catalysts. The utility of nanocatalysts (Pd, Ni, Ru, Ce, Cu, etc.) immobilized on biodegradable and recyclable supports (cellulose and chitosan) or on magnetic ferrites via ligands such as dopamine or glutathione fulfills most of the green chemistry principles and helps produce functional chemicals, which may find large-scale use, with extremely low levels of waste. See also: Catalysis; Influence of nanostructural features on the properties of magnetic nanoparticles





