The response of geologic materials to the environment (physical, chemical, and biological) at or near the Earth's surface. This response typically results in a reduction in size of the weathering materials; some may become as tiny as ions in solution.
Types
The agents and energies that activate weathering processes and the products resulting therefrom have been classified traditionally as physical and chemical in type. In classic physical weathering, rock materials are broken by action of mechanical forces into smaller fragments without change in chemical composition, whereas in chemical weathering the process is characterized by change in chemical composition. In practice, the two processes commonly overlap, almost inseparably. For example, diminution in particle size facilitates chemical reactivity, and an increase in volume of the products during chemical reaction may physically disintegrate the reactants.
Viewed broadly, environments of weathering and the suite of products from each may be categorized in terms of climate, such as desert, arctic, or tropical rain forest. In cold and dry climates, physical weathering predominates and produces angularity in both rock particles and surficial landforms. In warm humid climates, chemical and biochemical weathering yields rounded rock masses, and hydrated and oxidized mineral compounds which may be developed at great depths.
Agents
Within each environment, specific agents of weathering may be recognized and correlated with the types of effects they produce. Important agents of weathering are water in all surface occurrences (rain, soil and groundwater, streams, and ocean); the atmosphere (H2O, O2, CO2, wind); temperature (ambient and changing, especially at the freezing point of water); insolation (on large bare surfaces); ice (in soil and glaciers); gravity; plants (bacteria and macroforms); animals (micro and macro, including humans). Human modifications of otherwise geologic weathering that have increased exponentially during recent centuries include construction, tillage, lumbering, use of fire, chemically active industry (fumes, liquid, and solid effluents), and manipulation of geologic water systems.
Products
Products of physical weathering include jointed (horizontal and vertical) rock masses, disintegrated granules, frost-riven soil and surface rock, and rock and soil flows.
Products of chemical weathering include many which have been widely adapted to important economic and technologic uses. Such products include the soil, and the clays used in making ceramic structural products, whitewares, refractories, various fillers and coating of paper, portland cement, absorbents, and vanadium. These are the relatively insoluble products of weathering; characteristically they occur in clays, siltstones, and shales. Sand-size particles resulting from both physical and chemical weathering may accumulate as sandstones.
After precipitation, the relatively soluble products of chemical weathering give rise to products and rocks such as limestone, gypsum, rock salt, silica, and phosphate and potassium compounds useful as fertilizers.
Products of weathering that occur in colloidal sizes, also important qualitatively and quantitatively, are included in the preceding listings.
Processes of chemical weathering
Chemical reactions involving water and gaseous O2 and CO2 are probably the most important or abundant weathering processes on Earth. In sharp contrast, on the Moon, which is devoid of such an atmosphere, there is essentially no hydration, oxidation, or carbonation. Aqueous dissolution of rocks and minerals is probably the simplest or most straightforward process of chemical weathering. Solution rapidly removes rock salt (NaCl) and gypsum (CaSO4 · 2H2O), but more slowly corrodes carbonate, silicate, and oxide rocks.
Hydrolysis
Water dissolves O2 and CO2 from the air (possibly 10 times more CO2 from soil atmosphere), enabling it to oxidize and carbonate, as well as to hydrolyze rocks susceptible to those reactions. For example, Fe in silicate minerals is oxidized to Fe2O3, thereby removing Fe from the silicate structure and disrupting that network and making it more vulnerable to further breakdown. Oxidizing water reacts with metallic sulfides to produce the several sulfur-bearing acids, among them sulfuric acid, which is a powerful weathering reagent in itself. The metal constituents of the original sulfides typically become hydroxides or oxides. Fumes containing SO2, Cl2, or F2 from combustion of coal, from smelters, or from industrial furnaces generally combine downwind with water vapor (humidity), rain, fog, or dew to form weathering-effective acids.
Aqueous dissolution of CO2 produces carbonic acid, which has acidic and complexing (carbonate) properties. Dolostone (dolomite) and limestone (calcite) are quickly dissolved as Ca and Mg bicarbonates in carbonic acid, possibly producing topographic sinkholes, caves, and other karstic features, in addition to erosionally lowering the surface of those rocks. Turbulent and rapid flow of water on carbonate rocks markedly increases the rate of dissolution. The less soluble quartz, chert, clay, or iron oxides contained in dissolving limestone are left behind. Monuments and other structures composed of limestone and marble are similarly attacked.
Silicate rocks are attacked primarily by hydrolysis in a general reaction as shown below,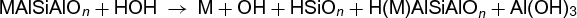
where M refers to metal cations (K, Na, Ca, Mg), subscript n denotes an unspecified ratio of atoms, and the Al following Si substitutes for . Thus there are formed, by hydrolysis, soluble alkali-metal hydroxides, soluble silica (the ionic distribution depends upon pH), and relatively insoluble clay mineral (or zeolite), or less commonly, hydrated alumina. If the hydrolysis takes place at pH 9.5 or higher, both silica and alumina will be relatively soluble and mobile. They may then be separated and form bauxite (Al2O3 · nH2O). Under more acid conditions, clay minerals are formed.
Adding hydrogen ions to the hydrolyzing system increases the rate of reaction. Carbonic acid, formed when the carbon dioxide of the air and soil dissolves in water, is a source of hydrogen ions which accelerate the reaction. Organic (humic) and other acids participate in the hydrolysis. Strongly complexing organic acids may mobilize (complex) in solution Al more effectively than Si from Al-silicate minerals. Solubilization in and precipitation from organic solutions are therefore sensitive to both Eh, the oxidation potential, and pH. Another major source of hydrogen ions is their production in the ionic atmosphere about the rootlets of growing plants. During plant growth and metabolism, hydrogen ions are evolved. These are exchanged by the roots for nutrient cations (K+, Ca2+, Mg2+) present in nearby clay colloids and rocks. Thus, the process of nutrition of plants is simultaneously a process of weathering of rocks. Hence, the energy which drives plant growth and is indirectly derived from the Sun likewise furnishes some of the energy for weathering of rocks.
Plant activity
Plants that are primitive in development apparently possess higher energies of cation exchange than do those that are more advanced. Lichens derive nutrient cations from fresh rock without intermediary soil. It is difficult to assess quantitatively the extent to which bacteria in the soil, and those coating interstices among mineral grains, accomplish chemical rock weathering, but some pedologists consider bacteria to be a major agent. See also: Soil microbiology
Rootlets of macroplants may sorb nutrients from adjacent soil when the mean free-bonding energy of the rootlet exceeds the crystal-bonding energy of mean free-bonding energies of clay minerals or organic substances by which they hold individual nutrient ions in polyionic systems in the soil. Hence, plant nutrition and the activity of agriculture occupy an intermediate position in the weathering sequence between fresh rock-forming minerals and intensely weathered “final” products of weathering (Figs. 1 and 2). Chelating organic substances extract cations from rocks, implementing rock breakdown. Partial weathering makes the rock constituents more available to plants, but extended weathering removes the nutrient materials entirely.
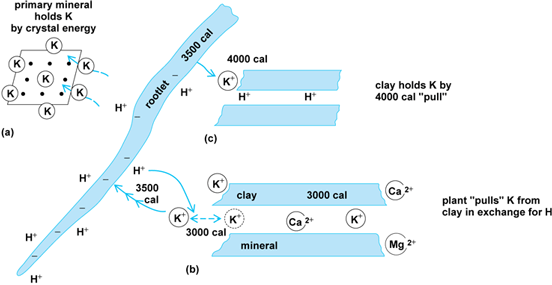
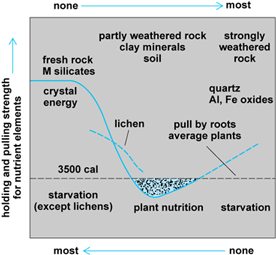
Results of chemical weathering
As shown by the hydrolysis reaction, the products from it may be broadly grouped into relatively soluble and relatively insoluble categories. The ultimate destination of the soluble products is the ocean, where they are concentrated in solution or removed by precipitation. Potassium released in solution by weathering, although as soluble as sodium, is more tightly sorbed by clay minerals and may be fixed in crystals of hydrous mica. Dissolved potassium is therefore less abundant than sodium in seawater. Magnesium may be incorporated in chloritic varieties of clay minerals. See also: Clay minerals
The most abundant weathering products of silicate rocks are the clay minerals. Weathering (hydrolysis) taking place in an environment such that high concentrations of calcium, magnesium, and iron (particularly ferrous) are built up tends to produce the smectite group of clays. Such a high concentration of ions occurs where evaporation exceeds precipitation, groundwater drainage is poor, or hydrolysis is rapid (as in weathering of volcanic dust). The kaolin group of clay minerals is developed where rainfall exceeds evaporation and leaching is intense. Oxidation of iron is then ordinarily high. Under conditions of very drastic leaching and continual wetting of the rocks, as in a tropical rain forest, silica and most cations dissolve, leaving hydrated oxides of alumina and ferric iron (bauxite and laterite). Rising groundwater solutions may carry Al and Fe upward and, because of evaporation or oxidation of organic complexes, leave deposits of both in the tropical subsoil. A high K+/H+ ratio in the aqueous-weathering system of Al-silicates yields the illite clay mineral (disordered K-mica). Weathering processes apparently reach a state of near-equilibrium with respect to kaolinite or smectite in environments such as those that prevailed where thick, valuable deposits of the clays were formed. In contrast, surface-exposed weathering of boulders and outcrops yields highly varied and changing products, quasimineral compounds, and rock wreckage.
Clay minerals, although relatively stable products of weathering in one environment, may be decomposed if subjected to more drastic leaching in another environment by processes of the removal of exchangeable cations, the more tightly fixed potassium of illite (hydrous mica) and possibly silica. Clay minerals are said to be degraded when their structures are partly destroyed. Entirely desilicated clays become bauxite or laterite. See also: Bauxite; Laterite





