Antisense drugs are gene-based molecules that inhibit the synthesis of proteins (including proteins that cause specific diseases) by binding to the ribonucleic acids (RNAs) responsible for their formation. Specifically, these drugs are single-stranded short polymers of RNA or deoxyribonucleic acid (DNA), termed oligonucleotides, designed to contain part of the noncoding strand of messenger RNA (mRNA), which is a molecule involved in translating DNA into protein. Antisense medications are therefore capable of hybridizing with and inactivating the mRNA, preventing the associated gene from producing the unwanted protein. With their anticancer, antiviral, and anti-inflammatory therapeutic capacities, these drugs have been applied in the treatment of various genetic disorders and infections, including diabetes, rheumatoid arthritis, cytomegalovirus retinitis (a virally caused form of blindness that occurs often in AIDS patients), asthma, hypercholesterolemia (a genetic derangement of fat metabolism characterized by very high levels of cholesterol in the blood), and numerous cancers. Research is also being conducted on patients suffering from Parkinson's disease and Huntington's disease to determine whether antisense therapy can mitigate the effects of these conditions. See also: Biotechnology; Deoxyribonucleic acid (DNA); Disease; Gene; Genetic engineering; Oligonucleotide; Protein; Ribonucleic acid (RNA)
One of the most intriguing applications of antisense drugs has dealt with the treatment of amyotrophic lateral sclerosis (ALS, also known as Lou Gehrig's disease). ALS is a progressive neurological disorder. It is characterized by the loss of connection and death of motor neurons in the cortex and spinal cord, resulting in paralysis, other motor deficits (including deficits in breathing, speaking, and swallowing), and death. Certain inherited forms of ALS are associated with mutations in the superoxide dismutase gene, and these mutations trigger overproduction of the superoxide dismutase protein. However, researchers have now applied antisense drug therapy in rodents to silence or turn off the superoxide dismutase gene, either by marking the genes for disposal or by halting the production of the superoxide dismutase protein. Moreover, the use of antisense drugs in rodent models with disease-causing mutations of the superoxide dismutase gene enabled these rodents to maintain muscle and nervous system integrity, as well as extending their survival. As such, permission has been granted to test antisense drugs in clinical trials with humans having ALS. See also: Degenerative neural diseases; Gene silencing; Muscular system disorders
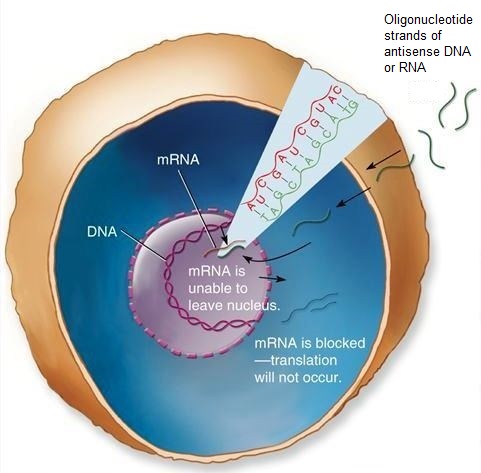
In general, an oligonucleotide is called antisense when it binds to complementary (sense) mRNA target sequences and blocks their translation into protein. The antisense oligonucleotide (or ASO) thus blocks or turns off gene expression with extraordinarily high specificity. Typically, this gene-silencing technique prevents the mRNA's message from being read by the ribosome (a cell organelle, present in large numbers in every living cell, that converts genetic information into protein molecules). Alternatively, the binding of the ASO to the target mRNA may activate a cellular enzyme (RNase H) that degrades and destroys the mRNA. The latter strategy has the advantage of preserving the antisense oligonucleotide so that it can continue to target other complementary mRNA sequences. Because antisense drugs are highly selective in their action, they are potentially less toxic than traditional drugs. They are also relatively quick to manufacture because the only information needed is the gene sequence. As such, antisense drugs can be rationally and rapidly designed to treat any disease that involves abnormal protein production, which gives them great promise in the fight against genetic conditions. See also: Enzyme; Molecular modeling for drug design; Pharmacology; Ribosomes





