In recent years, researchers have made considerable progress toward producing renewable energy in the form of hydrogen fuel through the electrolysis of water. In the most basic electrolytic cell, an electric current drives a chemical reaction at two electrodes in contact with an electrolyte solution to split water (H2O) into hydrogen (H2) and oxygen (O2). A more sophisticated photoelectrochemical cell under development, called an artificial leaf, splits water by using a silicon solar cell to absorb light and generate an electrical current and two electrocatalyst-coated surfaces to drive the same reaction. In all cases, efficient water splitting requires pure water to avoid damaging the electrodes. See also: Catalysis and catalysts; Electrochemistry; Electrolysis; Electrolyte; Hydrogen; Oxygen; Progress in developing an "artificial leaf" for hydrogen fuel generation; Solar cell; Water
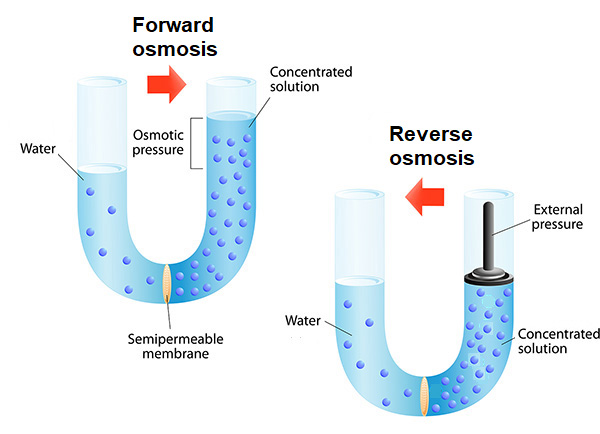
A commonly used technique to purify water is reverse osmosis, whereby pressure is applied to the surface of an impure water solution, forcing pure water to pass from the solution through a membrane (typically hollow fibers of cellulose acetate or nylon), leaving salts and other impurities behind. However, reverse osmosis requires significant energy to force water through the membrane, adding to the total cost of the hydrogen produced. Researchers reporting in Proceedings of the National Academy of Sciences of the United States of America (March 2021) took an approach called forward osmosis—a water separation process that uses a semipermeable membrane to separate water from dissolved impurities. Forward osmosis relies on osmotic pressure to transport pure water through the membrane, thereby reducing additional energy costs. See also: Membrane separations; Osmosis; Salt (chemistry)
In their forward osmosis experiment, the researchers enclosed an electrolytic cell in a simple plastic tube containing electrodes and a sodium phosphate electrolyte, with an opening at the bottom which they covered with a semipermeable cellulose acetate membrane. They immersed this tube in a saltwater solution and then applied an electric current to initiate the water-splitting reaction. As H2 and O2 production advanced, the cell became depleted in water, forming a concentration gradient that caused pure water to pass through the membrane to balance out osmotic pressure on both sides of the membrane. The experiment was both elegant and practical, demonstrating the potential for using nearly any water source for the water-splitting reaction without a costly purification step. The approach of using forward osmosis and water splitting together brings researchers one step closer toward the practical production of renewable hydrogen fuels. See also: Electric current





