Key Concepts
A device that converts light energy directly to electrical energy; also known as a photovoltaic (PV) cell. PV cells are made of various semiconductor materials, such as silicon and gallium arsenide. Solar cells may be used individually to power small applications, such as calculators, or connected in series and parallel as modules or panels to obtain the required values of current and voltage for electric power generation (Fig. 1). Connecting solar cells in series increases the voltage; connecting them in parallel increases the current. Modules may be grouped in parallel or strung in series to form PV arrays. See also: Electricity; Electric power generation; Electric power transmission; Energy; Light; Semiconductor; Solar energy; Sun

Photovoltaic effect
The conversion of sunlight into electrical energy, or the photovoltaic effect, involves three major processes: absorption of the sunlight in the semiconductor material; generation and separation of free positive and negative charges to different regions of the solar cell, creating a voltage in the solar cell; and transfer of these separated charges through electrical terminals to the outside application in the form of electric current. See also: Absorption of electromagnetic radiation; Electric charge; Electric current; Photovoltaic effect
In the first step, the absorption of sunlight by a PV cell depends on the intensity and quality of the sunlight, the amount of light reflected from the front surface of the solar cell, the semiconductor band-gap energy [which is the minimum light (photon) energy the material absorbs], and the layer thickness. Some materials such as silicon require tens of micrometers of thickness to absorb most of the sunlight, while others such as gallium arsenide, cadmium telluride, and copper sulfide require only a few micrometers. See also: Photon; Silicon
When light is absorbed in the semiconductor, a negatively charged electron and positively charged hole are created. The heart of the solar cell is the electrical junction which separates these electrons and holes from one another after they are created by the light. An electrical junction may be formed by the contact of: a metal to a semiconductor (this junction is called a Schottky barrier); a liquid to a semiconductor to form a photoelectrochemical cell; or two semiconductor regions (called a pn junction). See also: Electron; Electron-hole recombination; Hole state in a solid
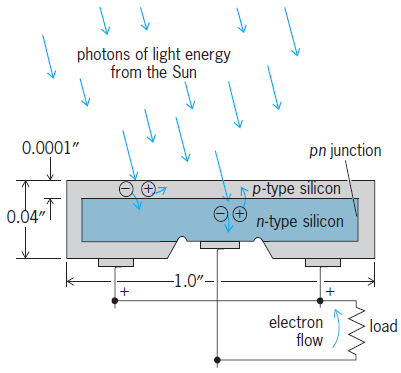
The fundamental principles of the electrical junction can be illustrated with the silicon pn junction. Pure silicon to which a trace amount of a fifth-column element such as phosphorus has been added is an n-type semiconductor, where electric current is carried by free electrons. Each phosphorus atom contributes one free electron, leaving behind the phosphorus atom bound to the crystal structure with a unit positive charge. Similarly, pure silicon to which a trace amount of a column-three element such as boron has been added is a p-type semiconductor, where the electric current is carried by free holes. Each boron atom contributes one hole, leaving behind the boron atom with a unit negative charge. The interface between the p- and n-type silicon is called the pn junction. The fixed charges at the interface due to the bound boron and phosphorus atoms create a permanent dipole charge layer with a high electric field. When photons of light energy from the Sun produce electron-hole pairs near the junction, the built-in electric field forces the holes to the p side and the electrons to the n side (Fig. 2). This displacement of free charges results in a voltage difference between the two regions of the crystal, the p region being plus and the n region minus. When a load is connected at the terminals, electron current flows in the direction of the arrow, and electrical power is available at the load. See also: Boron; Phosphorus; Semiconductor diode
Applications
Although the photovoltaic effect was discovered by French scientist Antoine César Becquerel in 1839 CE, practical solar cells made of silicon crystals were not developed until 1955. Beginning with Vanguard 1, launched in 1958, silicon solar cell arrays have become the almost exclusive power source for satellites. See also: Satellite (spacecraft); Space power systems
Solar cell arrays were initially used primarily to power small remote electrical loads that would otherwise be impractical or uneconomical to power by conventional means such as storage batteries or motor-generator sets. As the costs of solar cells fell dramatically and their efficiencies improved, solar cells were scaled up as large arrays that power facilities or feed into a utility grid. The first of these so-called solar parks or farms at megawatt-scale were built in the 1980s in the United States. For residential solar energy systems, PV modules and arrays can be mounted on a structural surface, such as a rooftop and faced toward the Sun (Fig. 3). When powering loads that require alternating current (ac) voltage, a power inverter is used to convert the direct current (dc) voltage from the solar cell array into usable ac power.






