Key Concepts
The division of a cell into daughter cells that receive identical copies of its genetic material. Cell division is the process by which living cells multiply (Fig. 1). The cell division cycle, more commonly and simply known as the cell cycle, comprises the period between the formation of a cell as a progeny of division and its own subsequent division into two daughter cells. The cell cycle can be divided into two parts. A relatively long interphase represents the time during which the cell engages in synthetic activities and reproduces its components, even though there is no visible change. The relatively short period of mitosis (nuclear division) provides an interlude during which the actual process of visible division into two daughter cells is accomplished. See also: Cell (biology); Cell biology; Cell cycle; Mitosis
Cell cycle
Prokaryotic and eukaryotic cells differ markedly in the coordination of deoxyribonucleic acid (DNA) synthesis and in the subsequent equal partitioning of DNA during cell division. In prokaryotes, the cell cycle consists of successive periods of DNA replication and of cell division in which a cell wall forms and divides the cell in two, with no visible condensation and decondensation of DNA. Eukaryotes from yeast to humans have similar cell division phases, termed G1, S, G2, and M phases. After each division, there is a time gap (G1 phase) before the synthesis of DNA begins. The cell is metabolically active during this part of the cycle in which proteins and DNA precursors are made. The period of DNA synthesis (S phase) involves the replication of chromosomal DNA; this is followed by another time gap (G2 phase), after which mitosis (M phase) occurs. Interphase is the time that elapses between one M phase and the next. It is composed of successive G1, S, and G2 phases and normally comprises 90% or more of the total cell cycle time. The cell cycle varies from tissue to tissue and during development. Sea urchin blastomeres, for example, replicate their DNA at the end of mitosis in telophase of the preceding division, thus eliminating the G1 phase entirely. See also: Chromosome; Deoxyribonucleic acid (DNA); Eukaryota; Prokaryote
The products of the series of mitotic divisions that generate the entire organism are called somatic cells. During embryonic development, most of the somatic cells proceed through the cell cycle. In the adult organism, however, many cells are terminally differentiated or no longer divide; they remain in a perpetual interphase. These cells are sometimes said to be quiescent or in G0 phase. See also: Cell differentiation; Developmental biology
Mitotic stages
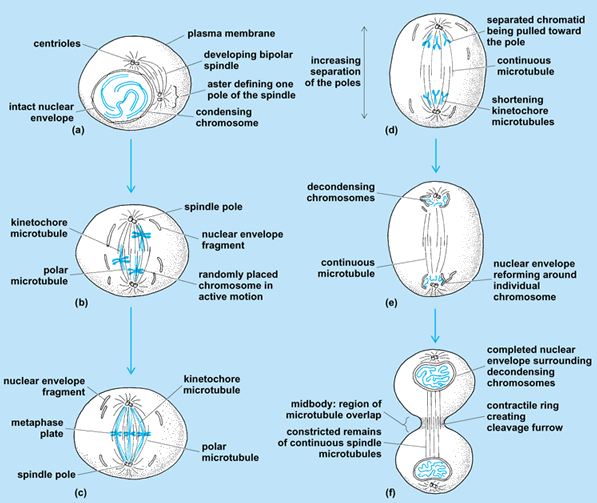
The mitotic or M phase of the cell cycle involves the separation of replicated nuclei into two identical daughter nuclei. Mitosis is divided into six distinct stages: prophase, prometaphase (or late prophase), metaphase, anaphase, telophase, and cytokinesis (Fig. 2). The length of time that a cell takes to complete mitosis varies in different organisms. In rapidly proliferating cells of higher eukaryotes, M phases generally occur only once every 16–24 h, and each M phase itself lasts only 1–2 h.
Prophase
Prior to mitosis, in the S phase of the cell cycle, chromosomal DNA replicates, and each chromosome divides into two sister chromatids. At the onset of prophase, these sister chromatids condense while remaining attached at a specific sequence of DNA necessary for chromosome separation called the centromere. At this point, the condensed chromosomes become visible by light microscopy. In interphase, the centrosome also divides, giving rise to two sets of centrioles. The centrioles, which are made from microtubules, also duplicate during interphase and begin to move to opposite sides of the nucleus to form separate poles. As these centrioles move apart, the microtubules disassemble. The new microtubules or asters, which will become intimately involved in the movement of the chromosomes during mitosis, begin to radiate from the centriole pairs in all directions to form the mitotic spindle. The number of microtubules making up the mitotic spindle varies, depending on the organism. The microtubules that connect at the center of the chromatid pairs are called kinetochore microtubules; others, called polar microtubules, will extend to the opposite pole. Astral microtubules radiate from the centriole pair outside the mitotic spindle. See also: Cell nucleus; Centriole; Centrosome; Mitosis and the spindle assembly checkpoint; Sister chromatid cohesion
Prometaphase
Prometaphase, or late prophase, begins with the breakdown of the nuclear membrane. At this point, the kinetochore microtubules interact with the chromosomes to form protein complexes at their centromeres. This association between the microtubules and proteins of the chromatids makes up the structure of the mitotic spindle. The microtubules that extend past the sister chromatids to each pole are stabilized through cross-linking of microtubule binding proteins. These microtubules are also protected from disassembly by the constant formation of new microtubules from tubulin molecules in the fluid portion of the cytoplasm (cytosol). The stabilization of these microtubules is necessary for mitosis, as shown by the effect of the drug colchicine, which blocks the assembly of microtubules and makes cells unable to complete mitosis. See also: Colchicine; Cytoplasm
Metaphase
At metaphase, the kinetochore microtubules align their chromosomes in one plane called the metaphase plate, half way between the spindle poles, with one kinetochore facing each pole. Each sister chromatid is now perpendicular to the opposite poles. The metaphase stage of mitosis is the longest, lasting up to 30 min in mammalian cells. The kinetochore microtubules align the sister chromatids by holding them in tension with the two opposite poles. This tension seems to be necessary for the correct movement of the chromatids in the next stage.
Anaphase
The sister chromatids are triggered to separate and migrate to opposite spindle poles, where they assemble to form the nucleus of a new cell. Anaphase is separated into two stages. During anaphase A, a sister chromatid pair separates at the kinetochore and is guided to an opposite pole as the kinetochore microtubules begin to shorten at a rate of 1 micrometer per minute. This separation occurs simultaneously for all the chromatids. As the chromatids reach the poles, the microtubules begin to disassemble. During anaphase B, the disassembling polar microtubules begin to elongate so that the two poles of the spindle move farther apart.
Telophase
The end stage of mitosis, termed telophase, begins with the arrival of the sister chromatids at each separate pole. As the kinetochore microtubules disappear, the polar microtubules continue to elongate. A new nuclear envelope begins to form around each group of sister chromatids. The chromatin that has been condensed throughout mitosis expands, and nucleoli appear. After this stage, the cell enters cytokinesis.
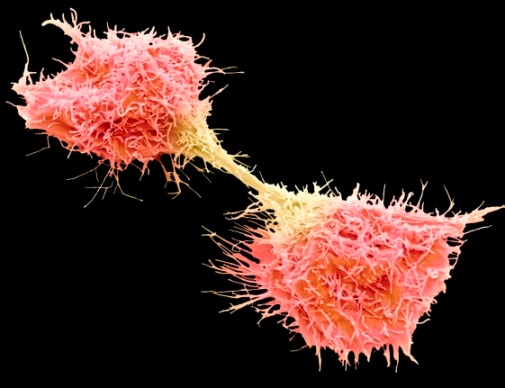
Cytokinesis
Cytokinesis begins during anaphase and ends the mitotic process. The cytoplasm divides by a process known as cleavage to give rise to two separate daughter cells. The forces required for cleavage are generated through actin–myosin interactions, which pull the plasma membrane down a furrow (a puckering of the plasma membrane). The cleavage furrow forms around the equator of the parent cell so that the two daughter cells produced are of approximately equal size and have similar properties. However, cytokinesis can sometimes fail, which is a condition termed incomplete cytokinesis. If cytokinesis is incomplete, the cleavage furrow degenerates and the two daughter cells do not separate, leading to a state in which the cell contains more than one nucleus. Experimental models have indicated that cytokinesis failure can contribute to the formation of tumor cells. See also: Cell membrane; Tumor
Induction of mitosis
The alternation between interphase and M phase is controlled by periodic changes in the activity of maturation promoting factor (MPF). This factor in amphibian eggs is present only in M-phase cytoplasm, and its activity is regulated by phosphorylations. Activity of MPF depends on two protein species associated in equimolar amounts in purified preparations. The first component is the protein kinase catalytic subunit p34cdc2. The second component is a 62-kilodalton protein that must be synthesized at each metaphase–anaphase transition in mitosis. Because of this cyclical behavior, this protein was identified as cyclin B; it is the MPF activator protein. There is a delay in an organism between the time after which protein synthesis is no longer required and the time when p34cdc2 kinase activation occurs. It has been suggested that the cyclin B accumulation required for MPF activation is not the only factor involved in the activation process. See also: Protein
Disruption
The order of the cell cycle is ensured by dependent relationships, that is, the initiation of late events depending upon the completion of preceding ones. Such dependence can be circumvented by pharmacological interventions, such as the induction of premature chromosome condensation by caffeine, blockade of mitosis and inhibition of microtubule formation by colcemid (demecolcine), and inhibition of cytokinesis by cytochalasin B (a mycotoxin). Mutations, which are more specific than inhibitions, also can involve molecules involved in cell division. Mutants and inhibitors aid in the identification of control mechanisms in cell division, named checkpoints, which govern mitotic entry and exit. See also: Mutation





