For the first time, scientists have observed two molecules meeting and reacting to form new molecules. A team of chemists used lasers to slow down interacting molecules to temperatures far colder than outer space, enabling the team to observe a chemical reaction colder than any on record or that could ever transpire in nature. The finding offers important insights into how chemical reactions transpire on a fundamental level, opening the door to a deeper understanding and potential control of reactions, which could subsequently be used to develop new materials and processes. See also: Chemistry; Laser; Molecule
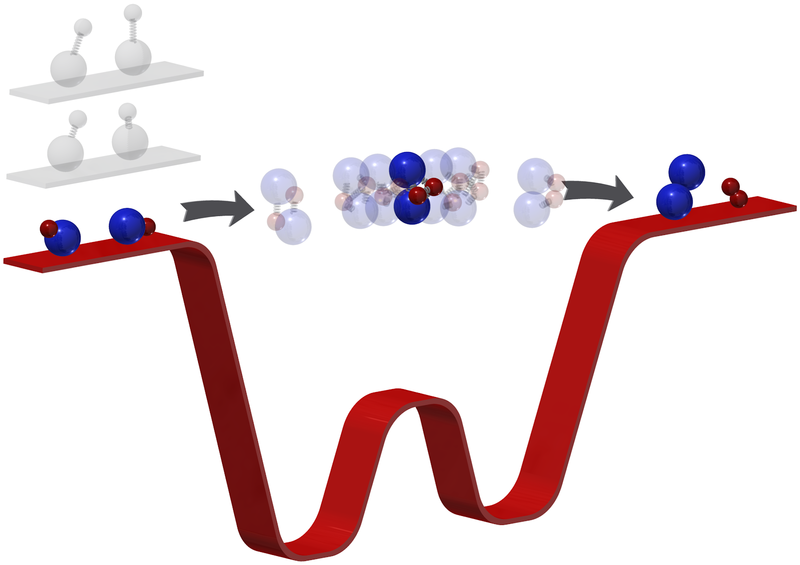
Normally, when two molecules collide and interact during a chemical reaction, an intermediate, conjoined molecule initially forms. This intermediate molecule then breaks apart in picoseconds, or thousandths of a billionth of a second—too fast for even state-of-the-art detector technology to definitively capture. By chilling a gas of potassium-rubidium (KRb) molecules down to 500 nanokelvin, or just a few millionths of a degree above absolute zero, chemists at Harvard University managed to sharply reduce the molecules' rotation, vibration, and motion—any of which allow for reacting molecules to quickly exit the intermediate phase. Thus trapped, the two KRb molecules persisted in the intermediate state—as a molecule of K2Rb2—for millions of times longer than in a usual chemical reaction, and well into the microsecond range. While still brief by most definitions, this period of time proved long enough for ultra-fast lasers, which act like cameras with rapid shutter speeds, to observe the reaction in unprecedented detail. The observations overall showed how the K2Rb2 molecule bonded and then subsequently cleaved into two new, single-element molecules: K2 and Rb2. See also: Absolute zero; Chemical bonding; Collision (physics); Potassium; Rubidium; Temperature
By learning how to manipulate molecules induced into an ultra-cold (cryogenic) state such as those in this investigation, chemical engineers could potentially control chemical reactions to devise new kinds of materials with novel properties. Further study could also offer new, precision probes of the forces involved in binding molecules together and ultimately splitting them apart. See also: Cryogenics; Intermolecular forces





