In a breakthrough in the global fight against malaria, the World Health Organization (WHO) is recommending widespread administration of the first-ever malaria vaccine. This first-generation vaccine is intended for children considered to be at high risk in specific regions where the disease is rampant, such as sub-Saharan Africa, Southeast Asia, and the Eastern Mediterranean. The WHO's announcement on October 6, 2021, is expected to significantly curtail the disease's impact, which is felt most acutely in sub-Saharan Africa, where malaria kills more than 260,000 children under the age of five annually. More than 200 million malaria infections occur globally every year (Fig. 1). See also: Infectious disease; Malaria; Vaccination
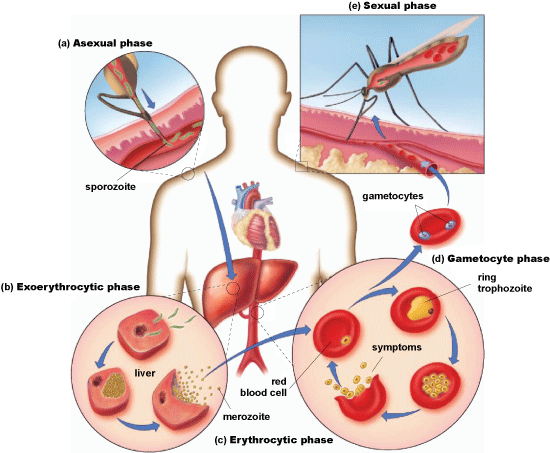
Malaria is caused by parasitic protists in the genus Plasmodium, a widespread group of single-celled organisms. These parasites enter the human body through bites from infected female mosquitoes in the Anopheles genus. The transmitted protists then travel from the bloodstream to the liver, where they mature and multiply before re-entering the bloodstream and infecting red blood cells (Fig. 2). Subsequent rupturing of the red blood cells frequently causes anemia. When a mosquito bites an infected human, the mosquito can ingest reproductive forms of the parasite that are circulating in the host’s bloodstream, which then travel to the mosquito’s salivary glands and the cycle will start anew in a new human host. People with malaria often experience periodic chills, fever, and sweats, as well as nausea and malaise. Severe illness and death can sometimes follow. Infants are especially vulnerable to severe illness and death. See also: Anemia; Blood; Liver; Medical parasitology; Mosquito; Protist; Zoonoses
Severe malarial disease is most often associated with infection by the species Plasmodium falciparum. Accordingly, scientists designed the new vaccine, called RTS,S/AS01, or RTS,S for short, to thwart P. falciparum. The pharmaceutical company GlaxoSmithKline had been developing an early version of the vaccine in collaboration with the Walter Reed Army Institute of Research since the 1980s. The new vaccine works by presenting virus-like particles to the human immune system, thereby eliciting the production of high levels of antibodies that trigger an immune response, consequently reducing the ability of the P. falciparum parasite to enter the liver. Scientists classify malaria vaccines by the targeted developmental stage of the parasite. RTS,S is considered a pre-erythrocytic vaccine because it targets P. falciparum parasites before they enter the liver. WHO is recommending that RTS,S be used in a schedule of four doses for the prevention of P. falciparum malaria in children living in regions with moderate to high transmission rates. See also: Antibody; Antigen-antibody reaction; Immunity; Immunology
Pricing for the RTS,S vaccine has not yet been determined, and researchers have labeled the vaccine’s efficacy as modest. However, an ongoing pilot program in the countries of Ghana, Kenya, and Malawi, in which more than 800,000 children had received the vaccine since 2019, had demonstrated the benefit conferred, leading to the recent WHO recommendation. A new clinical study that focused on a subpopulation of around 680 children in sub-Saharan Africa showed that, in combination with the usual seasonal administration of antimalarial medications that help reduce the spread and severity of disease, the vaccine lowered clinical malarial presentations—including hospital admissions with severe disease, as well as deaths—by approximately 70%. Clearly, a public health milestone has been reached as the first-ever vaccine against malaria to be tested in Phase 3 clinical trials is currently in implementation studies. Next steps will include involvement of the global health community in funding decisions and also decisions by countries on whether to adopt the vaccine as part of national malaria control strategies. See also: Africa; Pharmaceutical chemistry; Public health





