Key Concepts
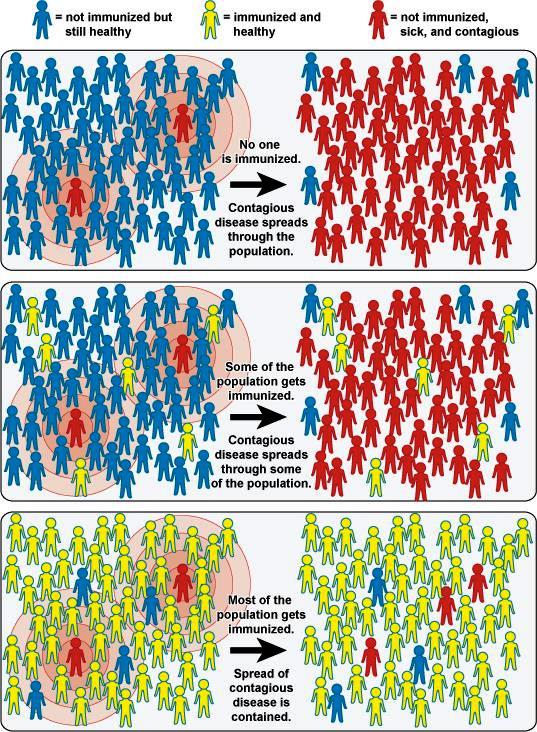
Active immunization against a variety of microorganisms or their components, with the ultimate goal of protecting the host against subsequent challenge by the naturally occurring infectious agent. The terms vaccination and vaccine were originally used only in connection with Edward Jenner's method for preventing smallpox, introduced in 1796. In 1881, Louis Pasteur proposed that these terms should be used to describe any prophylactic immunization. Vaccination now refers to active immunization against a variety of bacteria, viruses, and parasites (for example, malaria and trypanosomes) [Fig. 1]. In particular, the worldwide COVID-19 pandemic, which began in late 2019, has highlighted the importance of development of safe and effective vaccines. See also: Biologicals; Cellular immunology; Immunity; Immunology; Searching for a COVID-19 vaccine
History
Over the course of more than 200 years, vaccination has controlled nine major diseases: smallpox, diphtheria, tetanus, yellow fever, whooping cough (pertussis), poliomyelitis (polio), measles, mumps, and rubella. In addition, vaccinations against influenza (flu), hepatitis B, pneumococci, and Haemophilus influenzae type b have made major headway against these infections. In the United States and other economically developed countries, where programs have been expanded to immunize virtually all children under 2 years of age, most vaccine-preventable diseases have been reduced by 95% or more from prevaccine highs. The dream of eradication has been fulfilled in the case of smallpox, and polio has nearly been eradicated (although isolated polio outbreaks still occur in a handful of countries). See also: Diphtheria; Haemophilus; Hepatitis; Influenza; Measles; Mumps; Polio eradication; Poliomyelitis; Rubella; Smallpox; Streptococcus pneumoniae (pneumococcus); Tetanus; Whooping cough; Yellow fever
Implicit within Jenner's method of vaccinating against smallpox was the recognition of immunologic cross-reactivity together with the notion that protection can be obtained through active immunization with a different, but related, live virus. It was not until the 1880s that the next immunizing agents, vaccines against rabies and anthrax, were introduced by Pasteur. Two facts of his experiments on rabies vaccines are particularly noteworthy. See also: Anthrax; Rabies; Virus
First, Pasteur found that serial passage of the rabies agent in rabbits resulted in a weakening of its virulence in dogs. For the next 100 years, Pasteur's empirical approach for attenuating the virulence of a live virus by repeated passages in cells of species different from the natural host remained the principal empirical method for developing attenuated-live-virus vaccines. During multiple passages in an animal or in tissue culture cells, mutations accumulate as the virus adapts to its new environment. These mutations adversely affect virus reproduction in the natural host, resulting in lessened virulence. Only as the molecular basis for virulence has begun to be elucidated by modern biologists has it become possible to deliberately remove the genes promoting virulence so as to produce attenuated viruses. See also: Gene; Mutation; Virulence
Second, Pasteur demonstrated that the rabies virus retained immunogenicity even after its infectivity was inactivated by formalin and other chemicals, thereby providing the paradigm for one class of noninfectious virus vaccine, the killed-virus vaccine.
Attenuated-live and inactivated vaccines are the two broad classifications for vaccines (see table). Anti-idiotype antibody vaccines and deoxyribonucleic acid (DNA) vaccines represent innovations in inactivated vaccines. Recombinant-hybrid viruses are novel members of the live-virus vaccine class produced by genetic engineering. See also: Deoxyribonucleic acid (DNA); Genetic engineering
| Vaccine type | Disease |
|---|---|
| Attenuated-live | Measles, Mumps, Polio (oral, Sabin), Rotavirus, Rubella, Tuberculosis (BCG), Varicella (chickenpox), Yellow fever |
| Inactivated killed | Cholera, Hepatitis A, Influenza (injection), Polio (IPV, Salk), Rabies, Typhoid, Whooping cough (whole-cell pertussis) |
| Subunit/Conjugate | Haemophilus influenzae type B, Hepatitis B (HepB), Human papillomavirus, Meningococcal diseases, Pneumococcal diseases |
| Toxoid (inactivated toxin protein) | Diphtheria, Tetanus |
Attenuated-live vaccines
Because attenuated-live-virus vaccines reproduce in the recipient, they provoke both a broader and more intense range of antibodies and T-lymphocyte-associated immune responses than noninfectious vaccines. Live-virus vaccines have been administered subdermally (vaccinia), subcutaneously (measles), intramuscularly (pseudorabies virus), intranasally (infectious bovine rhinotracheitis), and orally (trivalent Sabin poliovirus). Combinations of vaccines have also been used. When combined live measles, rubella, and mumps vaccines have been given by injection, the antibody response to each component has been comparable with the antibody response to the individual vaccines given separately. See also: Antibody
Many organisms produce their pathophysiologic effects through acute infections localized to the gastrointestinal, pulmonary, nasopharyngeal, and genitourinary surfaces. These areas are bathed in mucus that contains highly important secretory immunoglobulin A (IgA). Subcutaneous, intravenous, and intramuscular immunization regimens, although effective in inducing IgG-type antibodies, are almost universally ineffective at the induction of secretory IgA antibodies. In contrast, oral administration is a feasible alternative to stimulate the common mucosal immune system, as with the live-attenuated polio vaccine. Live-virus vaccines administered through a natural route of infection often induce local immunity, which is a decided advantage. However, in the past, attenuated-live virus vaccines have been associated with several problems, including reversion to virulence, natural spread to contacts, contaminating viruses, lability, and viral interference. See also: Immunoglobulin; Virus classification; Virus interference
Noninfectious vaccines
Noninfectious vaccines include inactivated killed vaccines, subunit vaccines, synthetic peptide and biosynthetic polypeptide vaccines, oral transgenic plant vaccines, anti-idiotype antibody vaccines, DNA vaccines, and polysaccharide-protein conjugate vaccines. With most noninfectious vaccines, a suitable formulation is essential to provide the optimal antigen delivery for maximal stimulation of protective immune responses. Development of new adjuvant and vector systems is pivotal to produce practical molecular vaccines. See also: Antigen
Killed vaccines
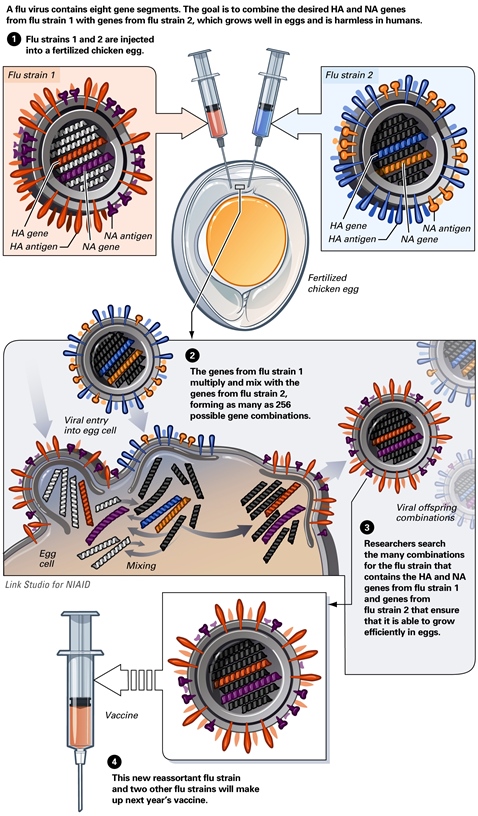
The inactivated forms of the toxins produced by the tetanus and diphtheria bacteria, called toxoids, were among the first vaccines developed. For human use, toxoid vaccines must be highly purified to remove extraneous materials. Killed-virus vaccines consist of partially purified virus particles whose infectivity has been destroyed by treatment with chemicals or with radiation. These virus particles retain the ability to elicit an immune response and to protect from clinical disease. Although generally safer and more stable than attenuated-live-virus vaccines, killed-virus vaccines require multiple doses of high concentrations of antigen sometimes administered with adjuvant, that is, a substance that enhances the potency of the antigen. The induction of cellular immune responses may be poor, and the protection induced by killed-virus vaccines may be of short duration, so booster vaccinations are needed. Vaccines produced by killing viruses have been known to contain surviving, infectious particles that have caused outbreaks of disease (Cutter polio vaccine and foot-and-mouth-disease vaccine). Although incapable of producing direct cellular destruction, noninfectious vaccines may elicit abnormal hypersensitivity reactions. The process involved in the production of an injectable killed vaccine is shown in Fig. 2; this example shows the steps involved in the flu gene reassortment that researchers carry out to create a proper flu vaccine.
Polysaccharide vaccines
Polysaccharide vaccines consist of the polysaccharide coats, or capsules, of encapsulated bacteria. Although polysaccharides are recognized mainly by T-cell-independent mechanisms, they do not effectively produce high-level, high-affinity antibodies, nor do they induce the T-cell memory required for booster responses. However, the immunogenicity of polysaccharides can be significantly increased by covalently linking them to carrier proteins. This approach is thought to work through the recruitment of T-cell help. For example, Haemophilus influenzae type b vaccines developed with this conjugate approach have been very successful in inducing high, boostable, protective antibody levels in infants and have dramatically reduced Haemophilus influenzae type b–associated disease in countries where they are in general use. See also: Polysaccharide
Subunit vaccines
Subunit vaccines consist of immunogenic viral proteins stripped free from whole virus particles and then purified from other irrelevant components, thereby reducing the risk of adverse reactions and residual infectious virus. Subunit vaccines require the addition of adjuvants or their formation into other forms [for example, lipid vesicles (liposomes and virosomes) or micelles]. The inability to propagate hepatitis B virus in tissue culture provided an incentive for the production of one of the first subunit vaccines, Heptavax-B, which was obtained by purifying and formalin-inactivating the surface antigen from the plasma of chronic carriers of hepatitis B. Experimental subunit vaccines have also been produced from purified glycoproteins derived from the virus membranes of enveloped virus particles, including parainfluenza-3, measles, rabies, influenza, respiratory syncytial virus (RSV), feline leukemia virus, bovine herpes virus-1, and pseudorabies. See also: Glycoprotein; Liposomes
Immune stimulating complexes (ISCOMs) are very immunogenic and represent an interesting delivery system for glycoprotein subunit vaccines. These complexes are really advanced liposomes that look like small balls and are made from an adjuvant.
Synthetic peptide vaccines
Chemically synthesized peptides are perceived as one alternative to conventional vaccines to elicit virus-neutralizing antibodies, or to “prime” the host for neutralizing antibody responses. A conventional approach to preparing antipeptide antibodies is conjugation of a peptide to a known protein or synthetic polymer carrier. Methods designed to avoid the use of carrier by polymerizing or cyclizing the peptides have also been reported, as well as an approach known as the multiple-antigen peptide system, in which a small peptidyl core matrix is covalently bound to radially branching synthetic peptides. See also: Broadly neutralizing antibodies; Neutralizing antibody
The peptide sequences chosen for synthesis in developing new vaccines correspond to antigenic determinants (epitopes) on the surface of virus particles that have been identified through chemical, electron-microscopic, and crystallographic analyses. Application of recombinant DNA technology has also allowed nucleotide sequences of viral genomes to be determined, from which primary amino acid sequences, secondary structure, and potential B-cell antigenic and T-cell (amphipathic) sequences may be deduced.
Carrier-free polypeptide vaccines are anticipated to be especially safe because they are chemically well defined, do not contain nucleic acids or extraneous proteins, and are unlikely to have any of the pathogens that might be present in serum or tissue extracts. Polypeptide vaccines are also extremely stable, withstanding thermal extremes and ambient conditions that might inactivate other types of vaccines under field conditions. This is important where target populations may lack access to refrigeration equipment.
Synthetic peptide vaccines may be feasible for agents for which protection is based mainly on humoral (antibody-mediated) immunity and suitable conserved antigenic sites can be found. Synthetic peptide vaccines are more realistic for DNA than for ribonucleic acid (RNA) viruses because DNA viruses mutate much less frequently than RNA viruses. Thus, antibodies against a single antigenic site of an RNA virus may allow too many escape mutants and, in contrast to DNA viruses, may require antibodies against different antigenic sites. Successful synthetic peptide vaccines have been produced for the simple, DNA-containing canine parvovirus and mink enteritis virus, which readily induce full protection of all vaccinated animals after a severe challenge with virulent viruses. See also: Canine parvovirus infection; Feline panleukopenia; Peptide; Ribonucleic acid (RNA)
Biosynthetic polypeptide vaccines
The nucleic acid sequences encoding microbial antigens and chemically synthesized peptides can be cloned in bacterial and yeast plasmids or in viral vectors and expressed efficiently in prokaryotic and eukaryotic cells, either as genetically purified (cloned) proteins or as parts of fusion proteins. The immunogens made in this way may be secreted into the medium or may be expressed on the surface or in the cytoplasm of cultured cells. The antigens can then be purified from the culture medium or from cell lysates. The biosynthetic production of antigens is particularly useful when the microbial agent is difficult to grow in tissue culture (for example, human hepatitis B virus) or dangerous [for example, rabies or human immunodeficiency virus (HIV)]. See also: Plasmid
Anti-idiotype antibody vaccines
The binding site of an antibody possesses a highly distinctive three-dimensional structure (the idiotype) that can itself act as an antigenic determinant to stimulate the formation of antibodies. The anti-idiotype antibodies, therefore, mimic the form of the surface antigen initially employed to induce the formation of a specific idiotypic antibody.
To prepare such vaccines, a monoclonal antibody is first produced specifically against a microbial antigen. This monoclonal antibody is injected into an animal to induce a second antibody specific to the monoclonal antibody. If the second antibody binds to the idiotype of the initial monoclonal antibody, then the antigen-recognition portion of the second antibody should be structurally analogous to the original microbial neutralization antigen. Injection of the second antibody as a vaccine will then induce antibodies against the original microbial agent. Experimental anti-idiotype antibody vaccines have been made against hepatitis B virus, rabies virus, HIV, herpesvirus, poliovirus, and several bacterial and parasitic pathogens. See also: Monoclonal antibodies
DNA vaccines
DNA (gene) vaccines, or the direct injection of DNA plasmids that express antigens of interest, represent another approach to subunit vaccines. In animal models, DNA vaccines stimulate cell-mediated and antibody-based immune responses, unlike most conventional inactivated vaccines. The DNA vaccines persistently express genes encoded by the plasmid without integrating into chromosomal DNA. Gene expression from the direct intramuscular injection of DNA has been detected in mice, rats, rabbits, chicks, dogs, fish, cattle, and nonhuman primates, illustrating the diversity of species in which direct DNA vaccination without a delivery vehicle is possible. DNA vaccines have a number of advantageous properties. They can protect against viral infections in which the antibody response alone is not protective, or where there is pronounced antigenic diversity among the target strains. Protection against lethal challenges has been achieved through parenteral or mucosal immunizations, or by the use of gene guns that deliver tiny amounts of DNA-coated gold beads. The same or similar DNA plasmids can be used as vectors for subsequent immunizations because no immune response is elicited to the vector. The development of new DNA vaccines may be pertinent to several diseases, including acquired immune deficiency syndrome (AIDS) and hepatitis B, and will undoubtedly receive increasing attention. See also: Acquired immune deficiency syndrome (AIDS); DNA vaccination
Adjuvants
Adjuvants are chemicals that significantly enhance speed, vigor, and persistence of strong antigens and the potency of weak antigens. Adjuvant formulations consist of adjuvants in suitable delivery vehicles, such as mineral or vegetable oil emulsions, squalene, liposomes, nonionic block polymer surfactants, and biodegradable polymer microspheres. Biodegradable microspheres can be especially useful as vehicles because of the ease of vaccine delivery by the oral route and the safety of the delivery vehicle (tissue compatibility and no secondary reactions). The most common microspheres range from a few micrometers (μm) to 200 μm in diameter.
The most common adjuvants used in immunological research are Freund's incomplete adjuvant, which consists of an aqueous antigen solution emulsified with an equal volume of paraffin oil/mannide monooleate, and Freund's complete adjuvant (muramyl dipeptide delivered in oil emulsions), which also contains killed mycobacteria. The complete adjuvant consistently stimulates high and long-lasting antibody responses, which can be attributed to the slow release of antigen from the site of injection. Neither form of adjuvant is acceptable for human use because both induce pain, abscess formation, granulomas, synovial joint lesions, and other adverse reactions. The only adjuvants authorized in the United States for human use are alum salts, which are both ineffective with some antigens and capable of producing adverse reactions, such as granulomas at the injection site. The emergence of subunit vaccines created by recombinant DNA technology and of synthetic peptide vaccines has intensified the need for safer and more effective adjuvants.
Cytokines and immunomodulation
The induction of an immune response in the presence of an appropriate antigen initiates reciprocal interactions between antigen-presenting cells, such as dendritic and Langerhans cells (and possibly macrophages), and T lymphocytes (T cells) bearing receptors for processed forms of the antigens. The development and propagation of the immune response also involves diverse subtypes of T cells and B lymphocytes, natural killer and lymphokine-activated killer cells, neutrophils, and eosinophils. The communications between these immune and inflammatory cells are mediated in large part by small proteins (about 20 kilodaltons) named cytokines. The cytokines include the interleukins (IL-1 to IL-18), gamma interferon, granulocyte-macrophage colony stimulating factor (GM-CSF), tumor necrosis factor, and transforming growth factor beta. The cytokines have various actions; for example, IL-1 is involved in T- and B-cell maturation, gamma interferon is involved in helper T cell 1 (Th1) upregulation and enhanced major histocompatibility complex (MHC) expression, IL-2 is involved in Th1 upregulation, IL-4 is involved in Th2 upregulation, IL-10 is involved in suppression of inflammatory cytokines and enhancement of B-cell proliferation, GM-CSF is involved in comigratory signals for dendritic cells, and IL-12 is involved in stimulation of Th1 differentiation. Cytokines are species-specific and expensive, and there are concerns regarding their stability, toxicity, and potential autoimmunity. These issues have limited their use as vaccine components. See also: Cytokine
Immunomodulation refers to the ability of many adjuvants to modify the cytokine network and, hence, the immune response. Adjuvants can target macrophages of the Peyer's patches (lymph nodules of the intestine) to induce mucosal immune responses, or they can allow antigens to associate directly with class I MHC molecules on the cell surface to elicit class I–restricted CD8+ cytotoxic T-lymphocyte responses. Particulate antigens tend to target macrophages, and macrophages can be recruited to the site of antigen deposition through the action of cytokines. Freund's complete adjuvant and lipopolysaccharide-based adjuvants drive the responses of Th1 subtype CD4+ helper cells, whereas alum salt, cholera toxin, and pertussis adjuvants drive the responses of Th2 subtype CD4+ helper cells. The Th1 responses are associated with interferon gamma, IL-2, and IL-12 secretions. Th1 cells also secrete tumor necrosis factor, thereby enhancing B-cell responses that elicit IgG2a (the antibody subclass most efficient in binding the serum complement proteins that enhance antigen-antibody reactions). The Th2 cells secrete the IL-4, IL-5, and IL-10 that activate the production of high levels of IgG1, IgA, and IgE by B cells. Selection of the appropriate immunoregulatory adjuvant not only leads to an enhanced immune response, but also determines the isotype of IgG, how much CD4+-directed cell-mediated immunity is generated, and which other immunoglobulins are made. See also: Antigen-antibody reaction; Immunologic cytotoxicity
Recombinant viral vectors and chimeric viruses
Another option is the use of recombinant viruses for the delivery of vaccine antigens. Although foreign antigens may be expressed in these recombinant viruses and used as inactivated subunit vaccines, live-attenuated-viral-based vectors as vaccines have decided advantages. This is because a single vaccination without adjuvants can suffice for immunization by the live-virus vaccine. Replication of the viral-based vector within the host amplifies the amount of foreign antigen being expressed, thereby often increasing both cell-mediated and humoral immune responses to the foreign antigen. The processing and posttranslational modifications of the foreign antigen within infected eukaryotic cells more closely resemble those occurring during natural infection with the pathogen from which the foreign antigen was derived. In addition, presentation of the antigen may be optimized.
Attenuated bacteria, such as Salmonella, as well as many different virus families have been used as vectors, including adenoviruses, herpesviruses, papovaviruses, retroviruses, and picornaviruses. However, the most extensively developed and exploited vector has been vaccinia virus, a member of the Poxvirus family. Vaccinia is a very large virus; about 5% of its DNA either is redundant or encodes genes that are not absolutely essential for virus replication in cultured cells. Thus, the capacity of vaccinia recombinants to accommodate foreign DNA is very large. Experimental vaccinia-based-vector vaccines include those with foreign DNA inserts derived from hepatitis B virus, influenza, malaria, Epstein-Barr virus, genital herpesvirus, respiratory syncytial virus, Lassa fever virus, human T-cell leukemia virus, and HIV. One of the most interesting of the vaccinia-based viral vectors is the recombinant that expresses the rabies virus glycoprotein gene. The vaccinia/rabies recombinant was incorporated in bait as an oral vaccine and is presently being used for the immunization of wildlife. Although vaccinia is a comparatively safe vaccine virus, immunosuppressed people can become systemically infected and die with generalized vaccinia infection. Therefore, it is difficult to imagine vaccinia being used in humans in view of the global AIDS epidemic. An additional problem arises from the induction of vaccinia antibodies during the initial usage of the vaccinia vector so that it becomes difficult to use the vectorial vaccine twice. See also: Animal virus
Recombinant DNA techniques also have made it possible to construct poliovirus type 1/type 3 antigenic hybrid viruses in cell cultures to elicit both type 1 and type 3 neutralizing antibodies. Three poliovirus serotypes are known, and each serotype displays three neutralizing antigenic sites. A type 1/type 3 chimeric virus constructed from clones of Sabin strain derivatives would be useful in the primary vaccination of children to reduce vaccine-associated cases of poliomyelitis.
Diarrheal disease vaccines
Diarrheal diseases are caused by the double-stranded, segmented RNA enteroviruses, by the single-stranded RNA Norwalk-type caliciviruses, and by enterotoxigenic bacteria (for example, Vibrio cholerae and Escherichia coli). These pathogens cause about 2.5 million deaths per year in children under 5 years of age, especially in countries where food and water are frequently contaminated. Inactivated vaccines and purified toxoids have traditionally been used to prevent cholera and other diarrheal diseases. Recently, however, genetic engineering techniques have enabled scientists to develop new oral vaccines. See also: Cholera; Diarrhea; Enterovirus; Escherichia
Rotavirus vaccines
Rotaviruses are the most common cause of severe diarrhea and dehydration in infants. In less economically developed countries, rotavirus diarrhea causes about 700,000 deaths per year. In the United States, about one-third of pediatric hospital admissions are the result of rotaviruses, with about 20 deaths per year. Rotaviruses elaborate an enterotoxin (nonstructural protein NSP4) that triggers a signal transduction pathway that alters epithelial cell permeability and chloride secretion. Natural protective immunity against severe rotavirus disease is built up during the first 2–3 years of life. Studies with live attenuated oral rotavirus vaccines have also shown that the majority of severe episodes of rotavirus diarrhea are preventable by oral immunization. In addition, several other candidate rotavirus vaccines, including bovine-human reassortants, are being investigated. See also: Infant diarrhea
Oral transgenic plant vaccines
Oral transgenic plant vaccines exemplify another innovative approach to vaccine development. Ultimately, the vaccine designers hope to use common, uncooked, edible plants, such as bananas and tomatoes, as transgenic plant delivery systems to express the antigens of pathogenic organisms in the gut, so as to induce secretory IgA, the predominant form of immunoglobulin found in mucosal secretions. Presently, however, only model studies have been completed using transgenic potatoes and tobacco plants; in these studies, human and animal diarrheal conditions were targeted to demonstrate the feasibility of oral transgenic plant vaccines.
Transgenic plants have many potential advantages as vaccines. In particular, they are easy to grow at minimal cost. Moreover, unlike bacteria and animal cells, they do not require specialist media and equipment or stringent purification protocols. Transgenic plants can be conveniently self-fertilized to produce stable, true-breeding lines propagated by conventional horticultural techniques and stored or distributed as seeds. Neither sterile syringes nor nurses would be needed for vaccination. See also: Plant breeding; Somatic cell genetics; Transgenic plant vaccines
Cancer vaccines
Materials commonly termed cancer vaccines are usually immunotherapeutic preparations targeted to increase the immune response to mutated cellular proteins. They are intended to inhibit the growth of previously diagnosed cancers rather than to prevent the appearance of new, spontaneous malignant tumors. However, in cases in which infectious agents are strongly associated with cancer induction, vaccines capable of preventing cancers have been made available. See also: Cancer; Oncology; Tumor; Tumor viruses
Viral hepatitis is a common infectious disease of humans. For example, there are 250–400 million carriers of the hepatitis type B virus (HBV) in the world, and it has been estimated that HBV causes about 1 million deaths worldwide per year. Carriers of HBV are at risk of developing hepatocellular carcinomas many years after the initial HBV infection. These carcinomas are quite prevalent in Asia, but various universal HBV vaccination programs have decreased their incidence.
Marek's disease virus is a cell-associated herpesvirus of poultry and causes a naturally occurring contagious malignant lymphoma in chickens. The disease has been effectively controlled using a live vaccine based on a nonpathogenic serologically related herpesvirus of turkeys. Cancers caused by other known infectious agents (including Epstein-Barr virus and hepatitis C virus) may also ultimately be preventable by vaccination. See also: Avian leukosis; Epstein-Barr virus; Hepatitis C virus





