Key Concepts
Any of the elementary infectious agents that possess some of the properties of living systems, such as having a genome and being able to adapt to changing environments. Viruses comprise a large group of infectious agents ranging from 10 to 400 nanometers in diameter. They are capable of infecting all animals, plants, and bacteria. However, because viruses are not functionally active outside their host cells, they are totally dependent on living cells for reproduction and metabolism. In general, viruses share three characteristics: (1) a simple, acellular organization consisting of a nucleic acid genome surrounded by a protective protein shell, which may itself be enclosed within an envelope that includes a membrane; (2) the presence of either deoxyribonucleic acid (DNA) or ribonucleic acid (RNA), but not both; and (3) an inability to reproduce independent of host cells. In essence, viruses are nucleic acid molecules, that is, genomes that can enter cells, replicate in them, and encode proteins capable of forming protective shells around them (Fig. 1). See also: Animal virus; Coronavirus; Deoxyribonucleic acid (DNA); Genomics; Infection; Nucleic acid; Ribonucleic acid (RNA); Virus classification; Virus infection, latent, persistent, slow
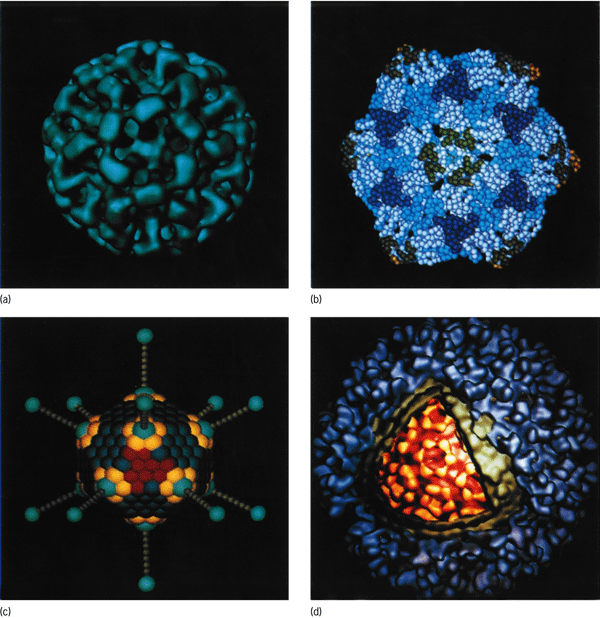
Recognition of viruses
One debate since the discovery of the chemical nature of viruses is whether or not these agents are living microorganisms. Lacking any metabolism, they can be considered as lifeless as isolated chromosomes. They are, however, capable of reproducing their own kind abundantly and, in that sense, possess one of the major attributes of life. Some scientists believe that this is sufficient reason to term them as living. Others, however, believe that these agents should not be referred to as living because they lack many of the basic abilities of life. It is preferable to refer to them as functionally active or inactive rather than living or dead.
The origin and evolution of viruses is a subject of much speculation among virologists. Two hypotheses have been proposed to account for their development. The first is that some of the more complex viruses, such as the poxviruses and herpesviruses, arose from small cells (probably prokaryotic) that parasitized larger, more complex cells. These parasitic cells then became simpler and more dependent on their hosts in a process known as retrograde evolution. There are several problems with this hypothesis. For example, viruses are radically different from prokaryotes; intermediate forms between the prokaryote and the complex viruses should still be in evidence, but they have not been found. The second hypothesis is that viruses are cellular nucleic acids that have become partially independent of the cell. Evidence supporting this hypothesis includes the observation that the nucleic acids of retroviruses contain sequences quite similar to those of normal cells. See also: Parasitology; Retrovirus
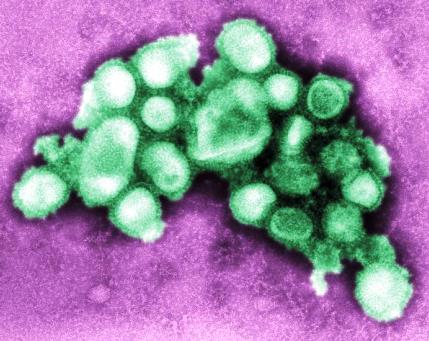
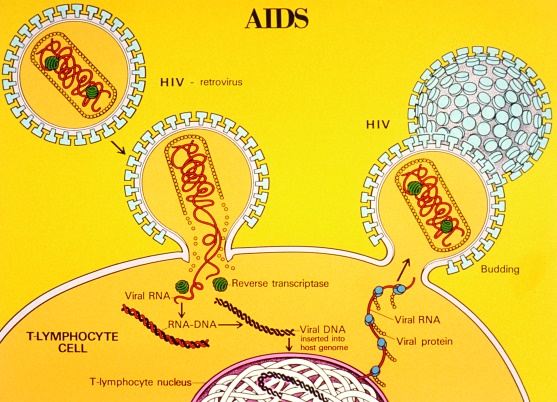
Viruses are recognized as significant causes of disease in animals and plants. Many of the most important diseases that afflict humankind, including poliomyelitis, hepatitis, influenza, the common cold, measles, mumps, chickenpox, herpes, rubella, hemorrhagic fevers, encephalitis, and the acquired immunodeficiency syndrome (AIDS), are caused by viruses. AIDS, first diagnosed in 1981 and caused by the human immunodeficiency virus (HIV), represents an unprecedented epidemic, with enormous implications for world health because case mortality rates for HIV-infected individuals are very high. Viruses also cause diseases in livestock [foot-and-mouth disease, avian influenza, swine flu (Fig. 2), and distemper] and plants (tobacco mosaic disease, tomato ring spot, and rice dwarf disease) that are of great economic importance. See also: Acquired immune deficiency syndrome (AIDS); Human immunodeficiency virus (HIV); Plant viruses and viroids
Viruses are also the simplest model systems for studying basic problems in biology. Virus genomes are often no more than one-millionth the size of, for example, the human genome, yet the principles that govern the behavior of viral genes are the same as those that control the behavior of human genes. Viruses thus afford unrivaled opportunities for studying mechanisms that control the replication and expression of genetic material. See also: Gene; Human genome
Morphology and size
Virus particles range in size from about 10 to 400 nanometers in diameter. The smallest viruses are about the size of a ribosome, whereas the largest, most complex viruses (for example, vaccinia) are visible when viewed in a light microscope. However, most viruses must be visualized using an electron microscope. Although viruses differ widely in shape and size, they are constructed according to certain common principles. Basically, viruses consist of nucleic acid and protein arranged in a structure known as the nucleocapsid. The nucleic acid is the genome containing the information necessary for virus multiplication and survival, whereas the protein is arranged around the genome in the form of a layer or shell that is termed the capsid. Some viruses consist only of a naked nucleocapsid. In others, the nucleocapsid is surrounded by a membrane, on the outside of which “spikes” composed of glycoproteins may be attached; this is termed the envelope. The complete virus particle is known as the virion (Fig. 3), which is a term that denotes both intactness of structure and the property of infectiousness. See also: Electron microscope; Glycoprotein
There are four general morphological types of capsids and virion structure. Icosahedral capsids are regular polyhedrons with 20 equilateral triangular faces and 12 vertices, appearing as spherical shapes when viewed at low power using an electron microscope. Helical capsids are shaped like hollow cylinders containing an extended viral nucleic acid. Enveloped viruses contain either helical or icosahedral capsids and have a roughly spherical shape overall. Complex viruses are neither purely icosahedral or helical, and they possess tails or other structures not found in simpler viruses.
Viral genomes
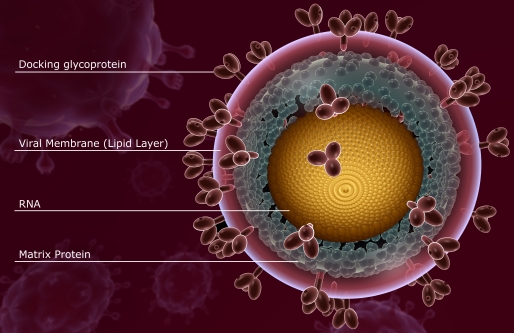
Viral genomes are astonishingly diverse. Some consist of DNA, whereas others consist of RNA (for example, HIV; Fig. 4). Some are double-stranded, whereas others are single-stranded. Some are linear, whereas others are circular. Some contain positive-sense RNA, meaning the genome can be directly read and translated into proteins, whereas others consist of negative-sense RNA and must be converted to a positive strand in order to be translated. Some consist of one molecule, whereas others consist of several molecules (up to 12). Their size also varies within wide limits: they comprise 3000 to 280,000 base pairs if double-stranded, and 5000 to 27,000 nucleotides if single-stranded.
Viral proteins
Viral genomes encode three types of genetic information. First, they encode the structural proteins of virus particles. Second, most viral genomes can be neither replicated nor decoded [that is, transcribed into messenger RNA (mRNA)] by host-cell enzymes. Therefore, most viruses encode enzymes capable of transcribing their genomes into mRNA molecules that are then translated by host-cell ribosomes, as well as nucleic acid polymerases capable of replicating their genomes. For example, the RNA genome of retroviruses encodes the enzyme reverse transcriptase, which is carried within the capsid when the virus infects a host cell. This enzyme allows these viruses to copy their genome and insert it into the host-cell chromosome, where it remains for long periods of time before it becomes activated by an outside stimulus. Many viruses also encode nonstructural proteins with catalytic and other functions necessary for virus particle maturation and morphogenesis. Third, many viruses encode proteins that interact with components of host-cell defense mechanisms against invading infectious agents. The more successful that these proteins are in neutralizing these defenses, the more virulent viruses are and the more severe the resulting disease. Larger viruses encode specific proteins with these functions. Smaller viruses do not; for them, these functions are exercised primarily by the structural proteins that they encode. See also: Enzyme; Reverse transcriptase; Ribosomes
Virus–cell interaction
The two most commonly observed virus–cell interactions are the lytic interaction, which results in virus multiplication and lysis (disintegration) of the host cell, and the transforming interaction, which results in the integration of the viral genome into the host genome and the permanent transformation or alteration of the host cell with respect to morphology, growth habit, and the manner in which it interacts with other cells.
Lytic interaction
The lytic virus–cell interaction is best thought of in terms of a cycle, the one-step growth cycle. This cycle involves the sequential, precisely regulated expression of the information encoded in the viral genome. The manner in which viral genomes express the information encoded in them is characteristic of each virus family.
The key features of the one-step growth cycle, which lasts, depending on the virus, from 6 to 36 h, are as follows. The parental virus particle adsorbs (attaches) to specific receptors located on the host-cell surface and is internalized by a process akin to phagocytosis. The viral genome is then either completely or partially released and expresses the information that it encodes by being transcribed into mRNA molecules (this is not necessary if the genome is positive-sense RNA), which are then translated. At the same time, the viral genome replicates either in the nucleus or in the cytoplasm, depending on the virus. Generally, DNA viruses replicate their genome in the nucleus, whereas RNA viruses carry out this process in the cytoplasm. When a sufficient amount of capsid proteins has accumulated, morphogenesis proceeds and progeny virus particles (up to 106 per cell for small viruses) are formed. Throughout this period, degradative and necrotic changes are elicited that result in the lysis of the cell. Naked viruses are released when the cells lyse; enveloped viruses are released when their nucleocapsids bud through the outer cell membrane, thereby acquiring their envelope. See also: Cell nucleus; Cytoplasm; Phagocytosis
When viruses infect multicellular organisms, the one-step growth cycle is repeated many times until, for one reason or another, further multiplication is arrested, or the host dies. The symptoms of virus infection vary widely, from asymptomatic infections detectable only by the formation of antibodies, to progressively more severe disease that can culminate in death. For every virus, there are variant strains that differ in the severity of their effects on cells and host organisms: the more severe the effects, the more virulent the virus strain is said to be.
Transforming interaction
Certain viruses interact with cells not only by means of the lytic interaction, but also by means of an interaction in which virus multiplication is repressed and the host cell is not destroyed. In this type of interaction, either the viral genome is integrated into the genome of the host cell (Fig. 5) or it replicates as a plasmid. The cells transformed by these viruses do not die and are capable of multiplying. Transformed bacterial cells are said to be lysogenic—they grow like normal cells; however, when the integrated viral genome that they harbor is activated, which happens with a frequency of about 1 in 105, a viral growth cycle ensues and the cells lyse. Transformed animal and plant cells are also capable of multiplying; they often grow into tumors, and the viruses that cause such transformation are known as tumor viruses. See also: Plasmid; Tumor; Tumor viruses
Tumor viruses are extremely important in studies on the mechanisms of cancer induction (oncogenesis) for two reasons. First, infection with tumor viruses generally transforms normal cells into potential tumor cells very efficiently; with all other tumorigenic agents, the frequency of transformation is much lower. As a result, studies at the biochemical and molecular level are possible on virus-induced transformation, but not on transformation caused by other agents. Second, elucidation of the mechanisms by which tumor viruses transform cells has identified the causes of carcinogenesis in molecular terms. See also: Cancer; Oncology
Viruses can cause cancer in several ways. A virus may bring oncogenes into a cell and insert them into its genome. For example, the retrovirus Rous sarcoma virus carries a gene that codes for tyrosine kinase. This enzyme is responsible for the phosphorylation of many cellular proteins that are regulated by the addition of a phosphate group. By bringing in a new source of phosphorylation, the regulation of these proteins is altered, and cell growth and behavior change as a result, potentially leading to cancer. See also: Oncogenes
Other oncogenic viruses carry one or more very effective promoters, which can then insert next to a cellular oncogene. This promoter then stimulates transcription of the oncogene, leading to cancer. For example, some chicken retroviruses cause lymphomas when their genome is integrated next to a cellular oncogene. This mechanism is known as insertional activation or proviral insertion.
A final mechanism involves the inactivation of proteins that negatively regulate cell division. The normal regulation of cell division involves the extremely sensitive interplay of proteins that either promote or inhibit it, depending on the signals received. Certain viruses, primarily DNA-containing viruses, encode proteins that bind or inactivate proteins that inhibit cell division. These viral proteins can thus result in the activation of cell division, promoting formation of cancer cells as well as stimulating virus reproduction. See also: Cell division
Antiviral chemotherapy
Because viruses enter host cells and make use of host-cell enzymes and constituents to reproduce, the development of drugs to treat viral infections seemed a remote possibility for many years. A drug that would block virus reproduction would likely be toxic to the host. However, inhibitors of virus-specific enzymes and life-cycle stages have now been discovered. Most antiviral drugs in use today disrupt either viral nucleic acid synthesis or specific stages in the virus life cycle. Viral nucleic acid synthesis is almost always carried out by virus-encoded enzymes that do not exist in uninfected cells and are therefore excellent targets for antiviral chemotherapy. Stages in the virus life cycle that have been elucidated through research are also used as targets in a number of antiviral treatments; these include enzymes required for attachment to host cells and enzymes responsible for virus maturation and release.
Numerous chemical compounds have been described that inhibit the multiplication of viruses. Only a few, however, inhibit virus multiplication efficiently in the body without undesirable side effects. The most successful of these compounds are analogs of ribonucleosides and deoxyribonucleosides. Another viral function that has been targeted is the cleavage of polyproteins, that is, precursors of structural proteins, to their functional components by virus-encoded proteases; examples include the HIV protease inhibitors used for treatment in AIDS patients. See also: Chemotherapy and other antineoplastic drug treatments; Virus chemoprophylaxis
Other promising approaches to antiviral chemotherapy include the use of agents that interfere with virus adsorption (primarily against HIV); agents that inhibit the enzyme neuraminidase, which is essential for the entry of influenza virus into cells and the subsequent release of its progeny from cells; and agents that bind in hydrophobic pockets of viral capsids, thereby stabilizing them and interfering with uncoating (primarily against picornaviruses, such as rhinoviruses). Additional methods include the targeted introduction of toxins into infected cells (against viruses for which the receptors are known, such as HIV, rhinoviruses, and Epstein-Barr virus) and the introduction into cells of specific antisense RNA sequences. Furthermore, oncolytic virotherapy is an emerging cancer therapy in which oncolytic viruses are used to target and destroy cancer cells without harming healthy cells. See also: Oncolytic virotherapy
Antiviral agents on which much interest is focused are the interferons. Interferons are cytokines or lymphokines that regulate cellular genes concerned with cell division and the functioning of the immune system. Their formation is strongly induced by virus infection; they provide the first line of defense against viral infections until antibodies begin to form. Interferons interfere with the multiplication of viruses by preventing the translation of early viral mRNAs. As a result, viral capsid proteins cannot be formed, and no viral progeny results. Interferon is clinically useful in the treatment of hepatitis B and C infections. See also: Antibody; Cytokine; Hepatitis; Hepatitis C virus; Immunology; Immunotherapy
By far, the most effective method of preventing viral diseases is through mobilization of the immune system by vaccines. There are two types of antiviral vaccines: inactivated and active attenuated. Most of the antiviral vaccines currently in use are of the latter kind. The principle of antiviral vaccines is that inactivated virulent or active attenuated virus particles cause the formation of antibodies that neutralize a virulent virus when it invades the body. See also: Vaccination





