Heat transfer is vital in many processes, including electric power generation, automotive propulsion and climate-control systems, household heating and cooling equipment, control of body temperature through clothing, thermal management of electronic equipment, and Earth-atmosphere systems, to name a few. Whenever there is a temperature difference, there is heat transfer.
Heat Transfer Enhancement
The three modes of heat transfer are conduction, convection, and radiation. Convective heat transfer—specifically the convective, or fluid flow-induced, process—can be enhanced or improved.
Consider the basic transfer equation applied to a heat exchanger:
Here q is the rate of heat transfer, U the overall heat transfer coefficient, A the surface area for heat transfer, and (Ta − Tb) the average temperature difference between the two fluids (or between the surface and adjacent fluid). The usual goals are to increase the heat transfer for a fixed area and temperature difference or to reduce the area (size) for a fixed heat transfer and temperature difference. Increasing the heat transfer coefficient, that is, enhancing the heat transfer, facilitates both goals. Passive enhancement techniques require no direct application of external power. Active enhancement techniques require external power.
Enhancement of convective heat transfer is one of the fastest- growing areas in heat transfer. Techniques for enhancing convective heat transfer include:
Passive techniques
Treated surfaces
Rough surfaces
Extended surfaces
Displaced enhancement devices
Swirl-flow devices
Coiled tubes
Surface-tension devices
Additives for fluids
Active techniques
Mechanical aids
Surface vibration
Fluid vibration
Electrostatic fields
Suction or injection
Jet impingement
Compound techniques
Rough-surface tube with a twisted-tape insert, for example
Many other possibilities exist when two or more techniques are combined (compound enhancement).
Single-phase convection
Single-phase free convection is found in such diverse applications as cooling of household refrigerator coils and cooling of electronic components. Single-phase forced convection is the most common type of convective heat transfer, used in household environmental control systems and industrial processes.
Passive enhancement
Passive techniques focus on the flow inside channels. The most common passive enhancement technique is to add fins or surface extensions. Interrupted fins are quite effective because they increase both U and A. Surface roughness has also been used extensively to enhance this type of heat transfer. Roughness and surface extension are usually combined, as most roughness also involves increasing the surface area. The real interest in extended surfaces is increasing heat transfer coefficients on the extended surface. Compact heat exchangers use several enhancement techniques such as offset strip fins, lanced fins, perforated fins, and corrugated fins. Heat transfer coefficients are several hundred percent above the smooth-tube values; however, the pressure drop is also substantially increased.
Internally finned (longitudinal or spiral) circular tubes are typically available in aluminum and copper alloys, but they also can be made in high-temperature materials such as silicon carbide. Correlations (for heat transfer coefficients and friction factors) are available for both straight and spiral fins. Improvements in computational techniques are expected, so that a wider range of geometries and fluids can be used. Examples of the wide variety of surfaces and inserts for tubes are shown in Fig. 1. Internal and external enhancement (double enhancement) is involved Fig. 1a, b, and d.
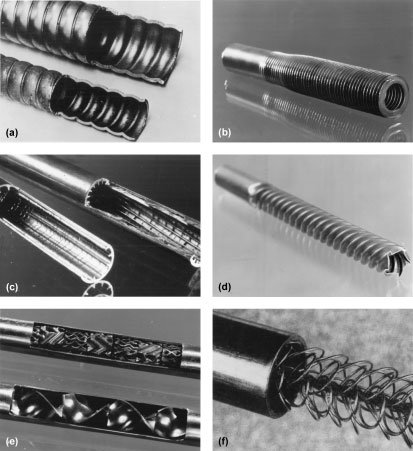
Many proprietary surface configurations have been produced by deforming the basic tube (Fig. 1a). A systematic survey of the single-tube performance of condenser tubes indicated up to 400% increase in the nominal inside heat transfer coefficient for water (based on diameter of a smooth tube of the same maximum inside diameter); however, pressure drops are as much as 20 times higher.
Displaced enhancement devices are typically in the form of tube inserts. In the tubes of a hot-gas-fired hot water heater, there is a trade-off between radiation and the turbulent convection. Wire-loop inserts have been used to enhance laminar and turbulent flow. Delta-wing and rectangular-wing promoters, creating vortices that co-rotate or counterrotate, have been studied.
Twisted-tape inserts have been widely used to improve heat transfer in both laminar and turbulent flow, and correlations are available. Several studies have considered the heat transfer enhancement of decaying swirl-flow, generated, say, by a short twisted-tape insert.
Active enhancement
Mechanically aided heat transfer in the form of rotation (stirring) or surface scraping can increase forced convection heat transfer. This is a standard technique in the chemical process and food industries when viscous liquids are involved.
Surface vibration has been demonstrated to improve heat transfer to both laminar and turbulent duct flow of liquids. Fluid vibration has been extensively studied for both air (loudspeakers and sirens) and liquids (flow interrupters, pulsators, and ultrasonic transducers). Although many studies have shown that heat transfer is improved when surfaces are vibrated (in some cases up to 10 times), this is not a popular technique because of possible equipment damage due to the intense vibrations. An alternative technique is used whereby vibrations are applied to the fluid and focused toward the heated surface. With proper transducer design, it is possible to improve heat transfer coefficients from simple heaters immersed in gases or liquids by several hundred percent.
A new development is a shape-memory-alloy coil that deploys at high temperature, when the heat transfer coefficient is low.
Some very impressive enhancements have been recorded with electrical fields, particularly in the laminar flow region. Electrostatic fields are particularly effective in increasing heat transfer coefficients in free convection up to 40 times, but with 100,000 volts. This technique has been studied extensively in the laboratory, and is used to cool cutting tools during machining. It is found that even with intense electrostatic fields the heat transfer enhancement is reduced as turbulent flow is approached in a circular tube.
Compound enhancement
Compound techniques hold particular promise for practical application because heat transfer coefficients can usually be increased more than is possible with any of the techniques acting alone. Some examples that have been studied for single-phase flow include rough tube wall with twisted-tape insert, rough cylinder with acoustic vibrations of the flow, internally finned tubes with twisted-tape inserts, externally finned tubes in fluidized beds, and externally finned tubes subjected to vibrations.
Boilers
Selected passive and active enhancement techniques have been shown to be effective for pool (static) and flow boiling.
Pool boiling
Surface material and finish have a strong effect on nucleate boiling (bubbles form at liquid-solid interface). However, reliable control of nucleation on plain surfaces is not easily accomplished. Accordingly, since the earliest days of boiling research, there have been attempts to relocate the boiling curve through relatively gross modifications of the surface. For many years, this was accomplished simply by area increase in the form of low helical fins (Fig. 2a). The subsequent tendency was to improve the nucleate boiling characteristics by fundamental changes in the boiling process. Many of these advanced surfaces, as depicted in Fig. 2, are being used in commercial boilers. Heat transfer coefficient increases of up to a factor of 10 are common with these surfaces. The advantage is not only a high nucleate boiling heat transfer coefficient, but also the fact that boiling can take place at very low temperature differences.
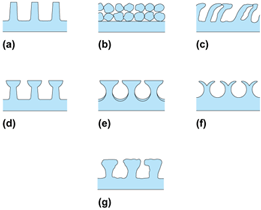
Active enhancement techniques include heated surface rotation, surface wiping, surface vibration, fluid vibration, electrostatic fields, and suction at the heated surface. Electrohydrodynamic enhancement was applied to a finned tube bundle, resulting in nearly a 200% increase in the average boiling heat transfer coefficient of the bundle, with a small power consumption for the field. Although active techniques are effective in improving heat transfer, the practical applications may be restricted, largely because of the difficulty of reliably providing the mechanical or electrical effect.
Compound enhancement has also been studied for pool boiling. Examples include fins and electric fields, electric fields in a bundle of treated tubes; radially grooved rotating disks; jet impingement on treated or rough surfaces; extended surfaces that are treated; and rough surfaces with additives.
Flow boiling
Helical repeated ribs, helically coiled wire inserts, and twisted-tape inserts have been used to increase in-tube vaporization coefficients in once-through boilers. Numerous tubes with internal fins, either integral or attached, are available for refrigerant evaporators. Original configurations were tightly packed, copper, offset strip fin inserts soldered to a copper tube, or aluminum star-shaped inserts secured by drawing the tube over the insert (Fig. 3a and b). Average heat transfer coefficients (based on the surface area of a smooth tube of the same diameter) for typical evaporator conditions are increased by as much as 200%.
A cross-sectional view of a typical micro-fin tube, now widely used for convective vaporization, is shown in Fig. 3c. The average evaporation-boiling coefficient is increased 30–80%, and the pressure drop observed is frequently lower.
Rotating flow can be generated by inlet swirl generators, twisted-tape inserts, or tangential injectors along the test tube. Twisted-tape inserts are generally used to increase the limiting or burnout heat flux for subcooled boiling at high imposed heat fluxes (107–108 W/m2 or 3 × 106 to 3 × 107 Btu/h ft2), as might be encountered in the cooling of nuclear fusion reactor components. Examples of compound enhancement in flow boiling include rough surfaces and additives, jet impingement with electrostatic fields, and electrostatic fields with micro-fin tubes.
Condensers
Vapor-space condensation usually occurs with the condensate flowing as a continuous film. Surface extensions are widely employed for enhancement of film condensation. The integral low-fin tubing (Fig. 2a), used for kettle boilers, is also used for horizontal-tube condensers. With proper spacing of the fins to provide adequate condensate drainage, the average coefficients can be several times those of a plain tube with the same base diameter. These fins are normally used with refrigerants and other organic fluids that have low condensing coefficients, but which drain effectively because of low surface tension. The fin profile can be altered whereby condensation occurs mainly at the tops of convex ridges. Surface-tension forces then pull the condensate into convex grooves, where it runs off. The average heat transfer coefficient is greater than that for a uniform film thickness.
Vapor-space condensation has also been enhanced by compound techniques, including coiled and fluted tubes, rotating and finned tubes, radially grooved rotating disks, and fins with electrostatic fields.
The applications of convective, in-tube condensation include horizontal kettle reboilers, moisture-separator reheaters for nuclear power plants, and air-conditioner condensers. Internally grooved or knurled tubes, deep spirally fluted tubes, random roughness tubes, and conventional inner-fin tubes have been shown to be effective for condensation of steam and other fluids.
The micro-fin tubes (Fig. 3c) have been applied successfully to in-tube condensing. As in the case of the evaporation, the substantial heat transfer improvement is achieved at the expense of a lesser percentage increase in pressure drop. By testing a wide variety of tubes, it has been possible to suggest some guidelines for the geometry, such as more fins, longer fins, and sharper tips; however, general correlations are not yet available. For heat-pump operation, the tube that performs best for evaporation also performs best for condensation.
Twisted-tape inserts result in rather modest increases in heat transfer coefficient for complete condensation of either steam or refrigerants. The pressure-drop increases are large, however, due to large wetted surfaces. Coiled tubular condensers provide a modest improvement in average heat transfer coefficient. The only example of compound enhancement for convective condensation is electrostatic enhancement of a micro-fin tube.
Benefits
Many heat transfer enhancement techniques have made the transition from the laboratory to industrial practice, where they typically result in halving equipment size or doubling heat transfer rates. Successful application depends on enhancement of the lowest heat transfer coefficient making up the overall coefficient U, including due allowance for surface fouling or corrosion. Some examples of systems that will have reduced weight and volume due to enhanced heat transfer are laptop computers, wearable computers, and space suits.
Finally, life-cycle costs must be considered. While enhanced surfaces or devices cost more initially, they often have short payback periods, less than 2 years, for example. The incentive to use enhancement technology is much greater now that the price of energy is increasing.
Interfacial Forces in Enhanced Phase-Change Heat Transfer
The use of interfacial forces to enhance heat transfer processes provides simple, passive, quiet cooling systems without mechanical pumps for small devices such as computers. These systems will be particularly useful in microgravity for the thermal control of satellites and space stations. In addition, the effective use of these forces leads to the optimization of many traditional processes.
Phase interfaces
Some characteristics of a liquid system, such as the vapor pressure at the interface, are a function of the intermolecular forces in the extremely thin boundary between the system and its surroundings. Due to the asymmetry of neighboring molecules, interfaces have intermolecular force fields different from those in a bulk solid, liquid, or vapor. Although the liquid-vapor interface is often shown as a two-dimensional surface without thickness, it is more accurately viewed as a diffuse dynamic interface with most changes occurring over a thickness of approximately 2 nanometers (8 × 10−8 in.) in which the molecules moving between the vapor and liquid have collisions and large velocities. A simple physical property that describes the average effect of this molecular asymmetry on the force field is surface tension. In a vapor, this interfacial tension (surface free energy) causes a small amount of liquid to form a spherical drop to minimize the free energy. On a solid substrate, sessile (attached) drops with different contact angles can form, depending on the surface free energies of the solid and liquid.
As the system becomes smaller, changes in the intermolecular forces at the interface become particularly important, and technologies are currently being developed that use these changes. In many cases, these additional changes can be related to the change in the surface shape.
Soap-bubble interface
The simple experiment of blowing a soap bubble shows that the pressure inside the bubble is greater than the pressure outside the bubble. Although this common system is more complicated than a pure liquid interface because it involves monolayers of soap molecules on both sides of a curved water film, the pressure jump can be shown to be a function of the radius of curvature of the bubble and its surface tension. The pressure on the concave side is greater than the pressure on the convex side and is inversely proportional to the radius of curvature. Very small bubbles give very large pressure differences because intermolecular forces are large. Measurement of the pressure jump and the radius of curvature gives the surface tension, which can be related to the intermolecular forcek field.
Length scales
Devices can be studied at the macro, micro, nano, molecular, or atomic scale. Based on the average volume occupied by a molecule in a bulk liquid, the average radius of a small molecule is approximately 0.3 nm (1.2 × 10−8 in.). The density of a liquid is approximately 1000 times that of a vapor at atmospheric pressure. Moving across the extremely thin interface between the liquid and vapor, the cohesive force per unit area due to intermolecular forces varies from the order of 3000 atmospheres in the liquid to essentially zero in the vapor. Since the larger part of this change in density occurs over a distance of approximately 2 nm (8 × 10−8 in.), large gradients in the intermolecular force field between molecules occur at interfaces. Large temperature gradients can also be generated within a small liquid system. Intermolecular forces are due to the fluctuating electrons in the various phases, and interfacial effects become asymptotically small at relatively large distances of the order of 0.1 micrometer (4 × 10−6 in.). On the other hand, they become very large at distances of the order of molecular dimensions.
Interfacial heat transfer
Some examples of heat transfer processes affected by interfacial forces are condensation or evaporation at a vapor-liquid interface such as the surface of an individual spherical drop in a vapor or a sessile droplet on a substrate, and adsorption of an ultrathin film on a solid substrate. Since interfaces have properties unique from those of a bulk liquid or vapor, use of these properties can lead to the development of enhanced heat transfer devices or simply the description of common physical phenomena. For example, since the shape-dependent vapor pressure of a curved interface or an adsorbed film is different than that of the bulk liquid, this difference can be used to control an evaporation/condensation heat transfer process in a small device without the need for an auxiliary mechanical pump. These systems are called passive since the fluids are pumped by intermolecular forces which are a function of the imposed temperature field.
Meniscus intermolecular properties
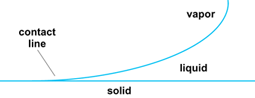
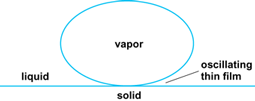
An important common system that has a gradient in the interfacial force field is the contact line region of a meniscus on a substrate where the liquid-solid-vapor phases meet (Fig. 4). At the leading edge of the curved film where the liquid film approaches the thickness of a monolayer of adsorbed vapor (in the case of boiling of a completely wetting liquid), the adsorbed molecules see only solid molecules on one side (at the liquid-solid interface). Farther along the liquid-vapor interface where the meniscus becomes thicker than approximately 0.1 μm (4 × 20−6 in.), the interfacial molecules interact with only liquid molecules on one side of the interface and only vapor molecules on the other side of the interface. Except for the effect of curvature at this thickness, this force field is similar to the interface of a bulk liquid. Therefore, the force field in the thicker film region is significantly different from that in the monolayer. If the intermolecular forces of cohesion between liquid molecules are larger than the forces of adhesion between the liquid and solid, a system with a finite contact angle will form. If adhesion is stronger than cohesion, complete wetting will occur. Since the local vapor pressure is a function of the shape, temperature, composition, and surface tension, the optimum use of these differences can lead to heat exchanger designs with enhanced performance. Use of intermolecular force concepts show that a superheat of the order of 40°C (72°F) is needed to remove the last monolayer of a simple fluid. Alternatively, theory shows that high rates of evaporation occur in the thicker region with negligible superheats at the liquid-vapor interface. In boiling, a large percent of the heat transfer occurs where the liquid between a vapor bubble and the solid substrate is thin (Fig. 5).
Heat transfer devices
For additional interfacial control, the vapor bubble can be constrained in a small container. A constrained vapor bubble (CVB) in a square glass cuvette (also called a wickless heat pipe) is presented in Fig. 6. Small-scale copper versions (micro heat pipes) are useful as passive heat exchangers for cooling computers. These systems are built by first pulling a vacuum and then partially filling the cuvette with liquid. The liquid preferentially fills the corners, forming a continuous meniscus in the axial direction. Energy input at the hotter end (1) vaporizes the liquid. The vapor then flows toward the colder end (2) and condenses along its length, where energy is removed due to heat loss. The shape-dependent interfacial pressure jump passively adjusts to give the necessary return flow of the condensate. Additional pumping capacity in a conventional heat pipe can be obtained by partially filling the cuvette with porous material which would give smaller radii of curvature. In the Earth's gravitational field, the shape of a large meniscus is distorted by gravity, and the usefulness of the device might decrease without the use of porous material. However, in microgravity the system can be larger without gravity affecting the performance. In fact, the viscous forces restricting the flow of liquid become less as the scale increases with a corresponding increase in the effective conductivity of the device. The passive evaporation/vapor flow/condensation mechanism in these devices can have an effective thermal conductivity 100–1000 times that of a bar of copper.

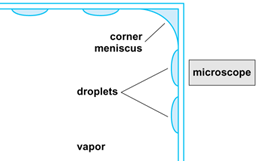
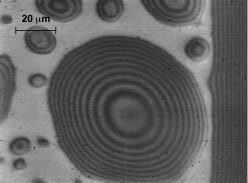
Ethanol vapor condensing on a glass substrate has an almost flat profile (that is, a small contact angle due to the strength of the intermolecular force field), making it easy to study. A not-to-scale schematic of the cross section of the CVB square glass channel with condensing ethanol is shown in Fig. 7. Looking at the condensation process through the wall of the container, using a microscope, shows a transient fringe pattern due to light interference (Fig. 8). The interference pattern gives the liquid thickness profile and, therefore, the force field. The uniformly bright region around the droplets indicates that only a very thin film of vapor is adsorbed in this region. The sessile droplets grow at a fast rate because the resistance to heat transfer is low in the regions where the liquid thickness is small. Once formed, the droplets are sucked into the corner of the CVB by capillary forces, thereby cleaning the surface for additional condensation. In small devices, the interfacial forces are larger than the gravitational force. Therefore, enhanced heat transfer can be obtained by having droplet condensation on a partially wetting surface instead of film condensation on a completely wetting surface. A finite contact angle can be obtained with ethanol by changing the molecular nature of the interface by heat-treating the glass. Proper use of these force fields lead to enhanced but simple passive heat transfer systems for high heat fluxes in small devices.
(A significant portion of the recent research described herein was supported by the National Aeronautics and Space Administration under Grants NAG3-1834 and NAG3-2351. Any opinions, findings, and conclusions or recommendations expressed herein are those of the authors and do not necessarily reflect the view of NASA.)
See also: Boiler; Boiling; Convection (heat); Film (chemistry); Heat exchanger; Heat pipe; Heat transfer; Intermolecular forces; Interface of phases; Laminar flow; Shape memory alloys; Surface tension; Turbulent flow; Vapor condenser





