Two new studies offer insight into why infection with SARS-CoV-2—the coronavirus that causes COVID-19—often leads to loss of smell, as well as why some people experience debilitating symptoms months after infection—a condition known as long COVID. See also: Coronavirus; The COVID-19 pandemic
The first study, published in the journal Cell, focused on cellular mechanisms behind loss of smell, or anosmia, in humans. In experiments with hamsters, biologists documented that SARS-CoV-2 preferentially infects and kills support cells, called sustentacular cells, in olfactory tissue lining the nasal cavity inside the nose. The immune system responds to the breakdown of sustentacular cells by sending in other cells and biochemicals, such as those that produce inflammation. Inflammation, in turn, affects olfactory sensory neurons—the cells chiefly responsible for detecting odors—that also reside in nasal cavity olfactory tissue. Then, olfactory neurons redirect their cellular activity and genetic expression away from the normal activity of olfaction to instead responding to the inflammatory signals. As a result, olfaction is severely impaired. See also: Cell (biology); Cell biology; Chemical senses; Hamster; Immunology; Inflammation; Neuron; Nose; Olfaction; Sense organ
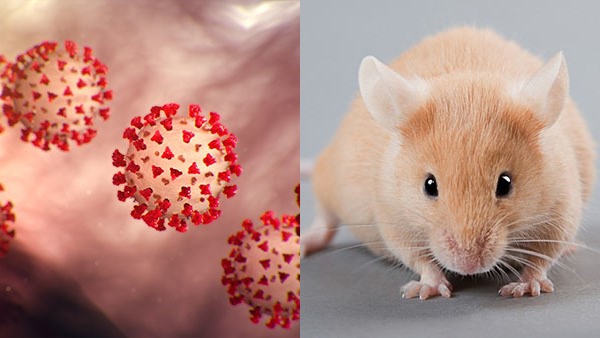
Typically, after a couple of days, inflammation subsides as the immune system clears out the debris from degraded sustentacular cells. The second study showed that, in certain individuals, this process can take longer, and—more problematically—the inflammatory response that initially began in nasal cells can thereafter occur upstream neurologically in the brain. Again, using hamsters infected with SARS-CoV-2, biologists obtained genetic sequences from the rodents' olfactory organs as well as regions of the brain including the prefrontal cortex, striatum, thalamus, cerebellum, and trigeminal ganglion. These genetic sequences indicated that in some hamsters, the cells in these areas of the brain showed evidence of ongoing inflammatory responses for weeks after the virus had been eliminated from their bodies. Hamsters with this prolonged inflammatory response demonstrated diminished cognitive performance compared to their conspecifics, consistent with symptoms of long COVID. See also: Brain; Cognition; Genetics
If these animal models are reflective of the human condition, the findings could have significant impacts on the treatment and management of long COVID. If the condition does indeed stem from an overreactive immune response, then dispensing anti-inflammatory medications such as steroids should prove effective for those suffering with this disease. However, scientists have not yet ruled out whether long COVID—or at least some cases of this condition—are instead caused by lingering, low-level infections of neurons in the central nervous system by SARS-CoV-2. If the latter, then steroid administration would be contraindicated, and immune-boosting medications or antivirals would likely be the appropriate therapeutic approach. The research team now plans to treat a long-COVID population of hamsters with steroids to determine if the medication helps the rodents return to normal activity. See also: Animal testing; Central nervous system; Pharmacology; Steroid





