Key Concepts
The set of reactions that use oxygen as the ultimate electron acceptor to produce adenosine triphosphate (ATP), generate heat, generate electrochemical gradients, and/or perform oxygen-dependent metabolic transformations. It is incorrect to equate cellular respiration (Fig. 1) with production of ATP (a vital energy compound in living cells) because some processes that produce ATP do not use oxygen and because some processes that use oxygen do not generate ATP. See also: Adenosine triphosphate (ATP); Biochemistry; Biological oxidation; Cell (biology); Cell biology; Electron; Energy metabolism; Metabolism; Oxygen
Biochemical background
In anaerobic systems, electron acceptors such as sulfate can substitute for oxygen. In contrast, glycolysis (the enzymatic breakdown of glucose and other carbohydrates) and many anabolic pathways do not utilize oxygen directly. However, if one tissue or microbe exports lactate from glucose fermentation to another tissue or microbe that fully oxidizes the lactate, the latter tissue or microbe is performing respiration for the former. Similarly, in the mitochondrial conversion of pyruvate to phosphoenolpyruvate that initiates gluconeogenesis (the formation of glucose from 3-C and 4-C substrates), a process that is not aerobic in the cytosol, oxygen is consumed in the production of nucleoside triphosphates needed for pyruvate carboxylase and phosphoenolpyruvate carboxykinase activities. Thus, in aerobic organisms, although not all metabolic transformation reactions are respiratory, aerobic respiration is essential for life. See also: Carbohydrate; Carbohydrate metabolism; Fermentation; Glucose; Lactate; Mitochondria; Respiration
The amount of free energy released from the complete oxidation of a fuel, such as glucose, to carbon dioxide (CO2) does not depend on whether the glucose is combusted in fire or by enzymatic processes. However, the ability of living things to convert fuels to ATP and to transform one set of molecules to other sets of molecules while also replicating and repairing their own machinery depends on redox (oxidation-reduction) enzymes and coenzymes. In a fire, the electrons go up in a smoke of CO2 and water vapor (Fig. 2). In living things, the energy is captured in processes that are largely optimized to minimize heat production. See also: Carbon dioxide; Coenzyme; Enzyme; Heat; Oxidation-reduction
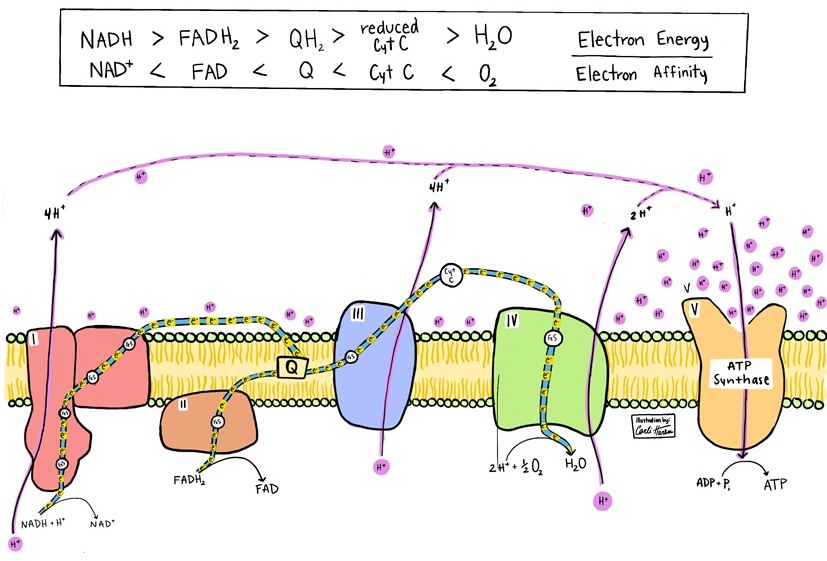
Thus, in cellular fuel consumption, high-energy electrons are captured onto nicotinamide adenine dinucleotide (NAD+), nicotinamide adenine dinucleotide phosphate (NADP+), and flavin adenine dinucleotide (FAD) in a process catalyzed by redox enzymes that oxidize substrates while generating reduced cofactors. The reduced coenzymes, that is, NADH, NADPH, and FADH2, are then able to donate electrons to redox acceptors of higher electron affinity. With each transfer to an acceptor of higher electron affinity, the energy of the electrons steps down (Fig. 1), and the energy released in these steps is used to generate electrochemical gradients, reduce disulfide bonds, and perform biosynthetic reactions. See also: Electron affinity; Nicotinamide adenine dinucleotide (NAD)
Respiratory electron transport chain
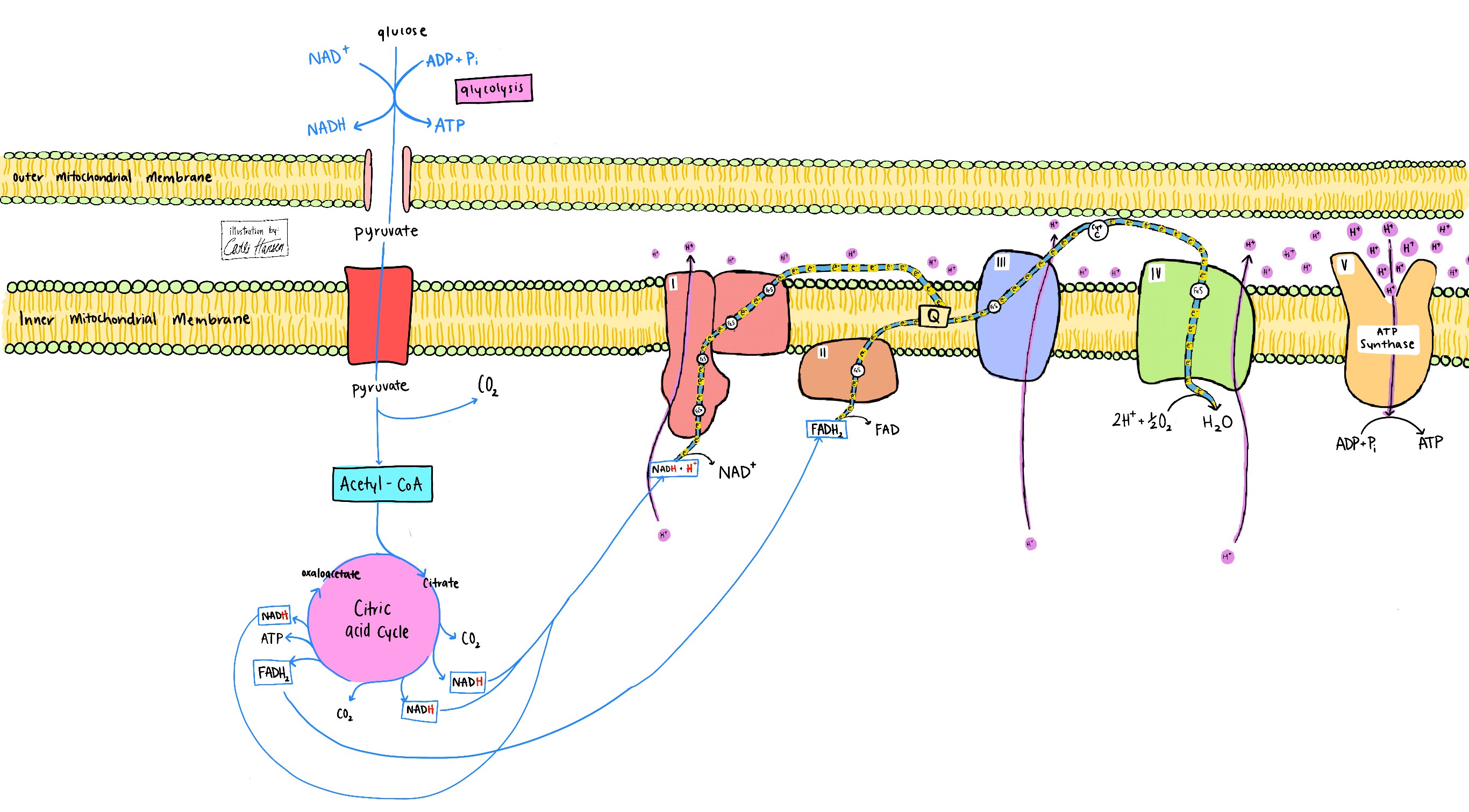
Mitochondrial inner membranes and plasma membranes of aerobic bacteria are the epicenters of cellular respiration. In these locations, the NADH and FADH2 are reoxidized to NAD+ and FAD with the electrons transferred to coenzyme Q, thereby forming QH2. For coenzyme Q to be ready to accept more electrons from reduced cofactors, electrons must flow to protein complex III. Similarly, complex III is ready to accept electrons on the basis of electron flow to complex IV, where four electrons and two protons are transferred from cytochrome C (a heme protein) to oxygen (O2), generating water (H2O). Thus, without O2 as the electron acceptor, mitochondrial fuel oxidation would have to be linked to an electron efflux system. The membrane-embedded respiratory complex—consisting of complexes I, II, III, and IV with diffusible NADH → NAD+, membrane-localized coenzyme Q, immobilized FADH2 → FAD, and diffusible O2 → H2O—is termed the respiratory electron transport chain. Each of the membrane protein complexes consists of many polypeptides with specifically located metal-containing redox-active centers that are intermediates in the chain (Fig. 1). See also: Bacteria; Cell membrane; Cytochrome; Protein
Process of cellular respiration
The overall process of cellular respiration can be likened to water flowing down a river that drives a turbine. Whereas building and maintaining the turbine are energy-dependent processes, the flow of water works with gravity so long as there is water upstream. Similarly, although producing and maintaining the mitochondrial enzymes, cell membranes, and cofactors are energy-dependent processes, fuel oxidation and respiratory electron flow are exothermic (that is, they liberate heat). Electrons flow in cellular respiration precisely as they flow in other electrical circuits, toward acceptors of higher electron affinity.
At the inner mitochondrial membrane, complexes I, III, and IV (which reoxidize the reduced forms of NAD+, coenzyme Q, and cytochrome C, respectively) utilize their higher electron affinities to pump protons across the membrane to generate a proton gradient. Just as the cost of turning a water turbine is paid for by water flowing downriver, the cost of pumping protons is paid for by electrons flowing from higher-energy states to lower-energy states. See also: Proton
Return of protons through the enzyme FoF1 ATP synthase generates ATP by oxidative phosphorylation (Fig. 1), whereas return of protons through proton pores (such as uncoupling proteins) generates heat (Fig. 3). Because electron affinity follows a gradient pattern of O2 > cytochrome C > coenzyme Q > FAD > NAD+, fuel oxidation typically transfers electrons first to NAD+ and/or FAD, and then to coenzyme Q, cytochrome C, and O2, with many additional protein-bound metal clusters as intermediates in complexes I, II, III, and IV.
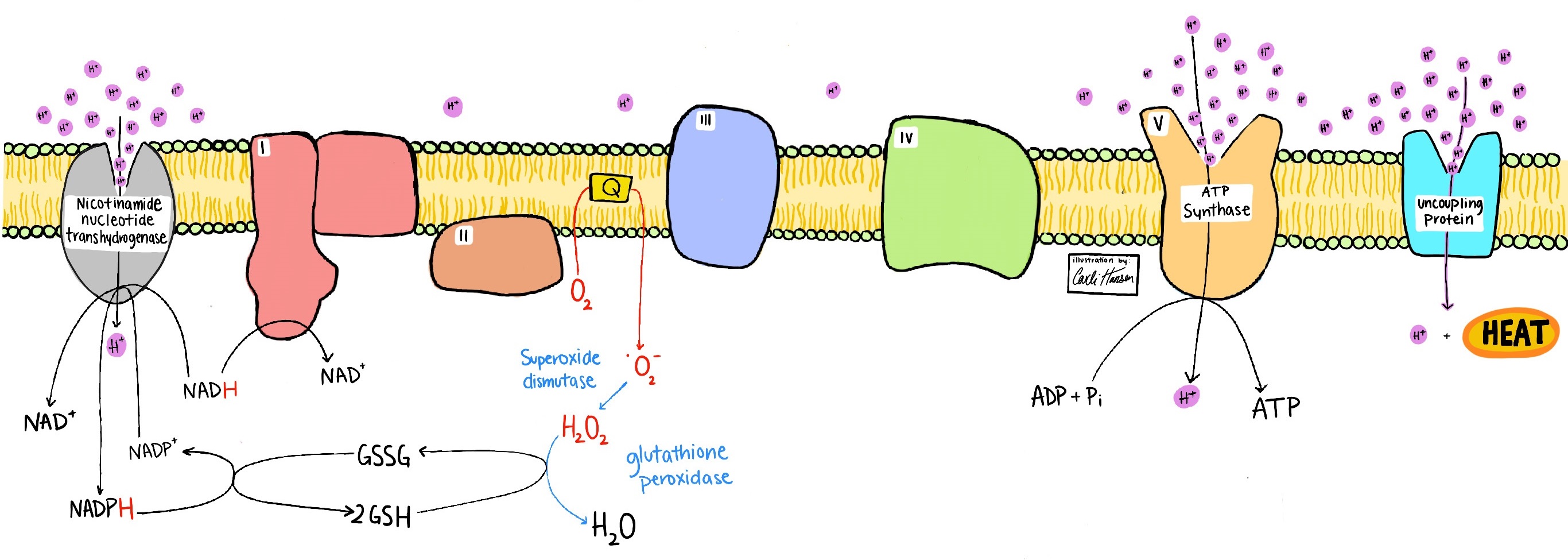
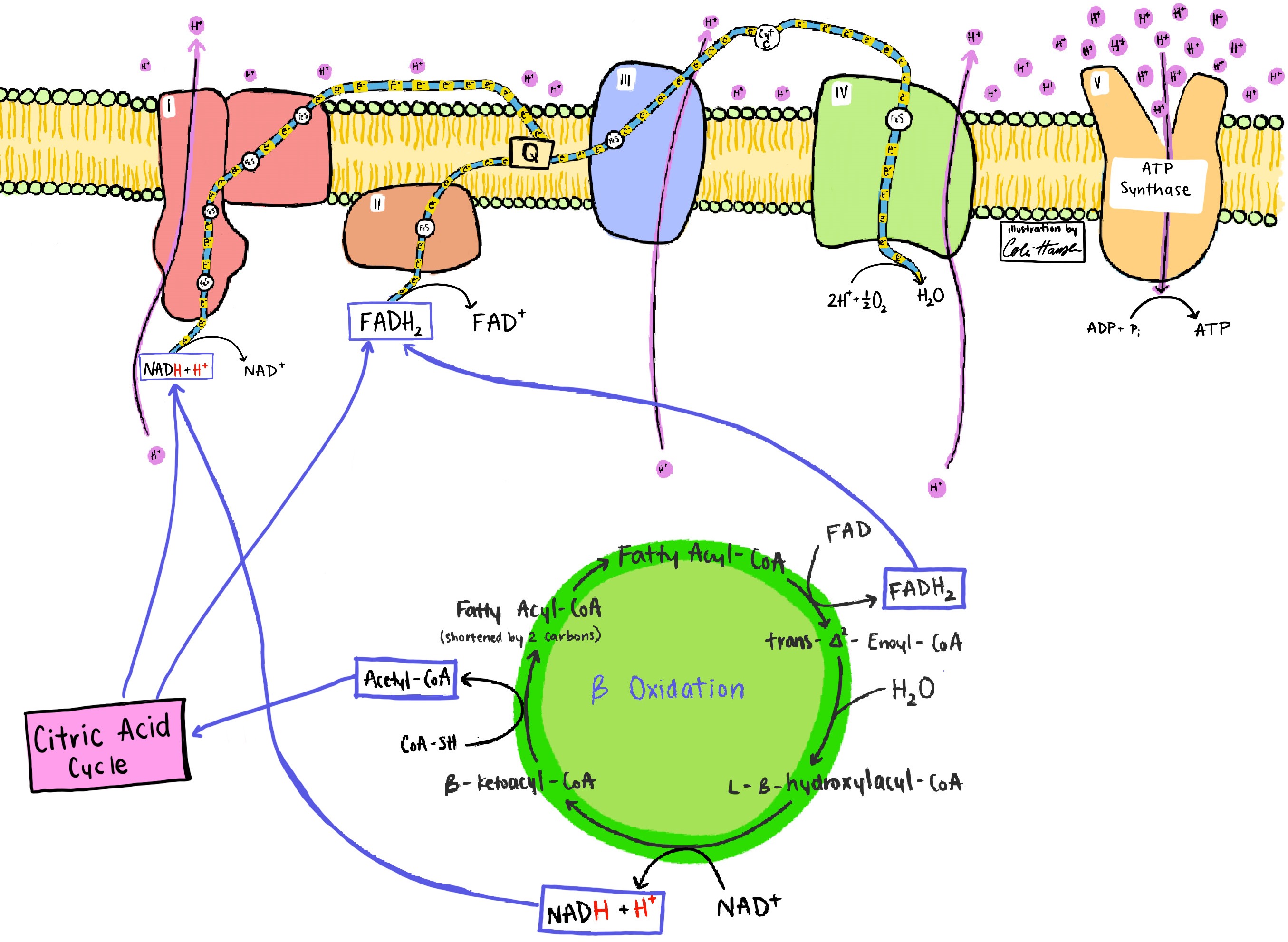
Small stepwise increases in electron affinity are manifested by small drops in electron free energy along the respiratory electron chain. The small differences serve to minimize heat production and to minimize production of superoxide (•O2−) and hydroxyl (•OH) free radicals. These reactive compounds are formed by collision between the radical form of coenzyme Q (•Q−) and O2 (Fig. 3). Damage produced by reactive oxygen species (ROS) is an obvious cost of aerobic metabolism, and ROS in the form of hydrogen peroxide (H2O2) and phospholipid hydroperoxides are controlled by glutathione reductases and glutathione peroxidases, which depend on NADPH as the reducing agent to reactivate oxidized glutathione. Thus, under conditions of ROS, there is a greater demand for NADPH for repair functions than for NADH for oxidative phosphorylation. Moreover, mitochondria have an enzyme termed nicotinamide nucleotide transhydrogenase (NNT) that can transfer hydride from NADH to NADP+ to generate the NADPH. Protons return through NNT in order to drive this catalytic process in a manner that is directly competitive with production of ATP and heat (Fig. 3). See also: Free energy; Free radical; Hydrogen peroxide; Superoxide chemistry
Respiratory demands vary by type of fuel, by the balance between catabolism and anabolism in which a cell is engaged, and by the degree to which the cell produces cytosolic NADPH anaerobically through processes such as the pentose phosphate pathway (in which glucose is metabolized or transformed into NADPH).
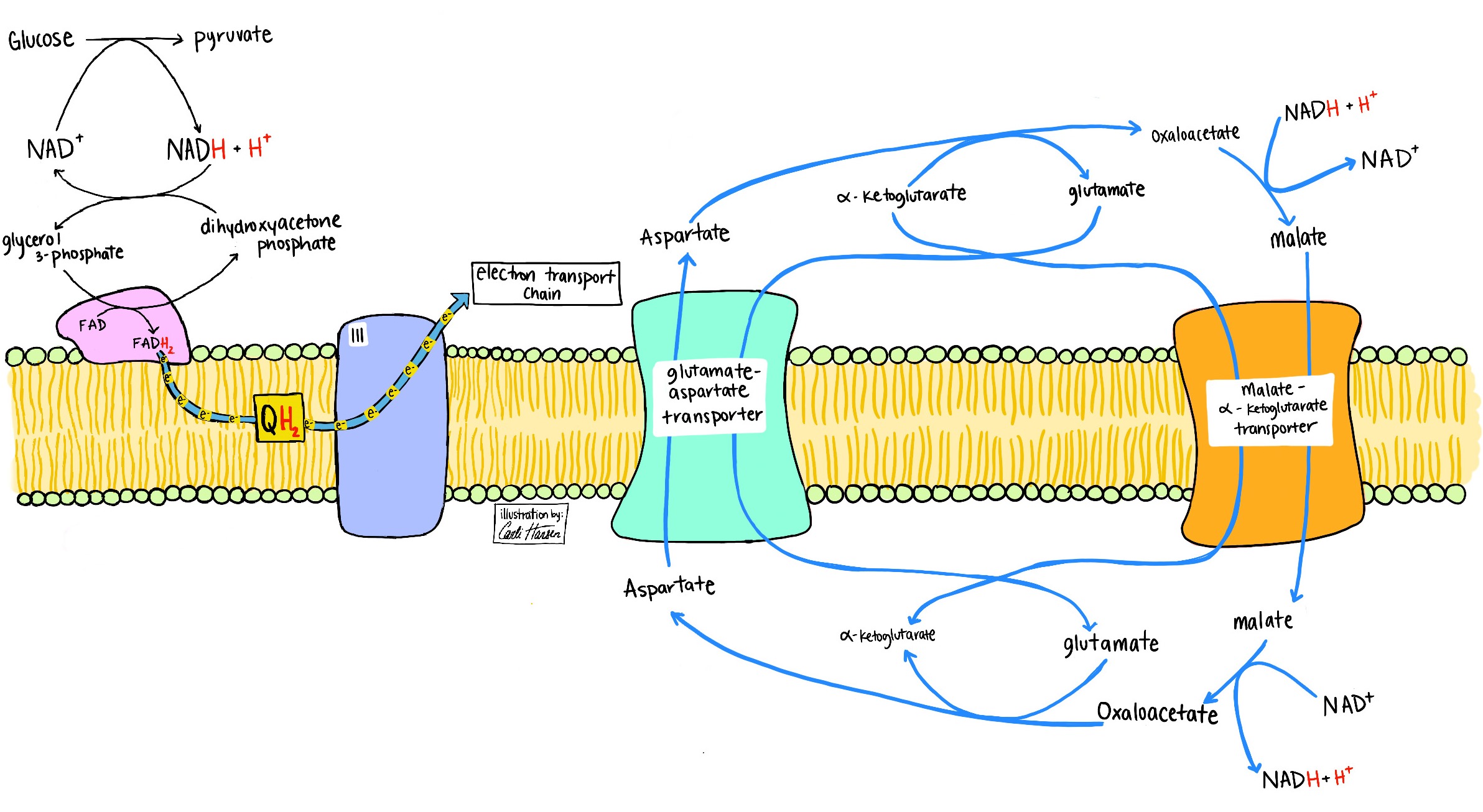
Whereas the process of glucose to pyruvate oxidation is anaerobic, the electrons captured on NADH in the glyceraldehyde phosphate dehydrogenase step can be used in oxidative metabolism by virtue of lactate transport to another tissue and/or transfer to mitochondria through other mechanisms, including the malate-aspartate shuttle and the glycerol-3-phosphate shuttle (Fig. 4). When pyruvate is fully oxidized to CO2 in mitochondria by the combined action of pyruvate dehydrogenase and the citric acid cycle (Krebs cycle), electrons are captured onto NADPH, NADH, and FADH2, necessitating O2 as the electron acceptor to reoxidize NADH and FADH2. NADPH formed by the action of isocitrate dehydrogenase (a mitochondrial citric acid enzyme) is used largely to control ROS that accompany aerobic reoxidation of NADH and FADH2 in the electron transport chain (Fig. 5). See also: Citric acid cycle
In contrast to glucose oxidation, the complete oxidation of triglycerides (neutral lipids consisting of three fatty acyl chains esterified to a glycerol backbone) is almost entirely aerobic (Fig. 6). Glycerol oxidation to pyruvate produces one cytosolic NADH anaerobically, while saturated even-numbered fatty acids that are oxidized to CO2 in mitochondria have all electrons captured on NAD+ and FAD, necessitating O2 as the ultimate electron acceptor. The ratio of fatty-acid carbons to glycerol carbons in a triglyceride provides an indication of how aerobically demanding triglyceride oxidation is. For example, in a triglyceride that contains three 16-C fatty acids, a single cytosolic NADH is generated per >140 reduced mitochondrial coenzymes generated in production and oxidation of the 48 fatty acid–derived acetyl-coenzyme A (Ac-CoA) molecules. Considering that the cytosolic NADH can be effectively reoxidized aerobically via the malate-aspartate shuttle or the glycerol-3-phosphate shuttle and that the glycerol-derived pyruvate can also be oxidized in mitochondria, complete oxidation of a typical triglyceride can demand sufficient oxygen to reoxidize approximately 150 mitochondrial NADH and FADH2 equivalents. See also: Lipid; Lipid metabolism; Triglyceride (triacylglycerol)
It should also be pointed out that amino acid oxidation is intermediate in its O2 requirement between glycolysis and mitochondrial fatty-acid oxidation because some reduced cofactors are produced in the cytosol and others are produced in the mitochondria. See also: Amino acid; Amino acid metabolism
The other consideration that guides the magnitude of a cellular O2 requirement is the degree to which a cell is busy with reactions that demand the hydride carried on NADH and NADPH and whether reducing equivalents can be produced cytosolically. Unlike a fireplace, whose purpose is to combust fuel fully to generate heat (Fig. 2), living things create and repair everything that they are made of, and carry out the work of resting and active metabolism from consuming foods.
Thus, the logic of life is such that the relatively low energy electrons carried on cytochrome C in the inner mitochondrial membrane have much less power to do meaningful work than the electrons carried on cytosolic NADPH. The former can donate to O2 to generate water, having already generated a proton gradient in the descent from the high-energy state in NADH to the low-energy state in reduced cytochrome C. The latter can donate electrons to beta-keto groups and alkenes to perform reductive biosynthesis. Therefore, it would be illogical for cells to let electrons flow downhill too far if they are needed for biosynthetic reactions.
Example of white adipocytes
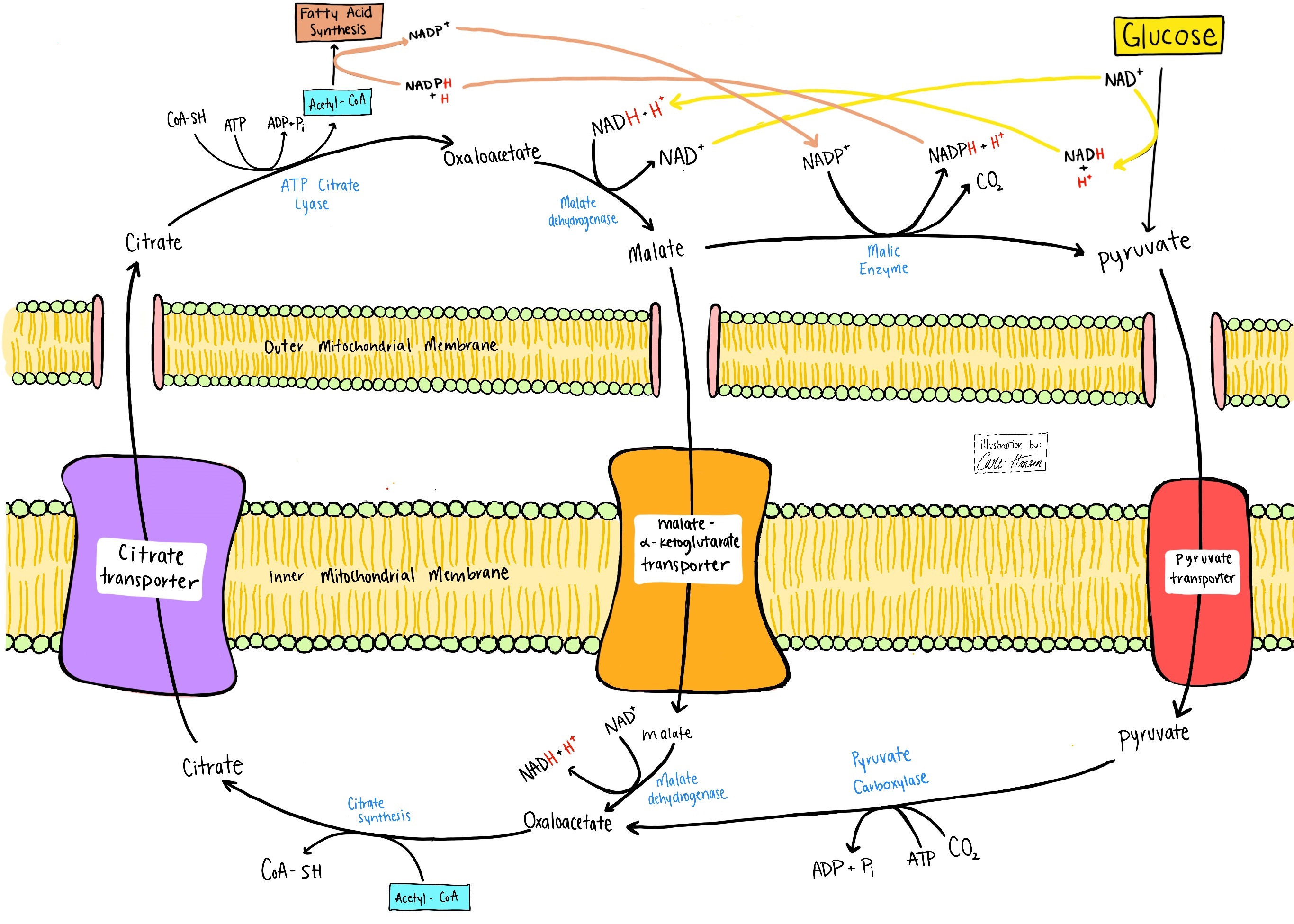
One of the best examples of a set of metabolic pathways that minimizes respiration occurs in white adipocytes (fat-storing cells), which are specialized to convert glucose to triglycerides (Fig. 7). This begins with import of glucose and conversion to pyruvate in the cytosol. In the mitochondria, pyruvate is converted to oxaloacetate and Ac-CoA by pyruvate carboxykinase and pyruvate dehydrogenase. These products are condensed to form citrate, which is then exported to the cytosol for conversion to cytosolic Ac-CoA and oxaloacetate. In an ATP- and NADPH-dependent process, the adipocyte runs de novo lipogenesis (fatty-acid synthesis) to produce palmitate (a 16-C saturated fatty acid). The adipocyte can return three of four carbons of oxaloacetate to the mitochondria as pyruvate in a process that reoxidizes cytosolic NADH for continued glycolysis (oxaloacetate → malate) and that generates a cytosolic NADPH for continued lipogenesis (malate → pyruvate). Only half of the pyruvate that enters mitochondria has to be oxidized to Ac-CoA with production of an NADH. The glucose-derived Ac-CoA is not oxidized to CO2 in the citric acid cycle, but rather is effectively exported to the cytosol to produce fat. Moreover, because the adipocyte cytoplasm can produce NADPH by running the oxidative and nonoxidative phases of the pentose phosphate pathway and by converting oxaloacetate to malate and then malate to pyruvate, it has a system to capture most of glucose's available electrons into fat synthesis without a high oxygen demand.
Other considerations
Although it is beyond the scope of this article to cover cell replicative and anabolic pathways, it is important to consider that every cell and tissue make everything in the human body from food using metabolic transformations whose biosynthetic complexities greatly exceed the catabolic complexities of breaking down carbohydrates, fats, and proteins. Thus, it is not merely in lipid synthesis that high-energy electrons captured by NAD+ and/or NADP+ are used to build things. Gluconeogenesis, ketogenesis, amino acid synthesis, nucleic acid synthesis, and steroid synthesis depend on reduced cofactors. In addition, NADPH is used to generate bursts of ROS by NADPH oxidases. See also: Fat and oil; Nucleic acid; Steroid
Like de novo lipogenesis, cytosolic ROS detoxification depends on NADPH that can be produced in the cytosol by nonaerobic processes, including the pentose phosphate pathway. However, when ROS are generated in mitochondria, the NADPH produced in the citric acid cycle could be insufficient for ROS detoxification and thus the NNT system is used to convert mitochondrial NADH to mitochondrial NADPH. Because NNT depends on proton return to transfer hydride from NADH to NADP+, the electron transport chain continues to consume oxygen. Similarly, in brown adipocytes, which express high levels of the proton pore–forming uncoupling protein, high levels of oxygen consumption are linked to heat production rather than ATP formation (Fig. 3).
Summary
In summary, cellular respiration encompasses the oxygen-dependent and electron transport chain–dependent processes by which coenzymes reduced by fuel oxidation are reoxidized. It is linked to mitochondrial ROS production and detoxification, generation of electrochemical gradients across membranes, thermogenesis, and oxidative phosphorylation. It is minimized by cells performing high levels of reductive biosynthesis, despite their ongoing fuel oxidation.





