Key Concepts
Electromagnetic field fluctuations that form quantized traveling waves and the manner in which they are created. Electromagnetic radiation is also referred to as electromagnetic waves, photons, and light (Fig. 1). Electromagnetic radiation consists of fluctuating electric field and magnetic field components that form self-propagating, traveling waves. As they travel, these waves transport energy, momentum, and information. These waves are fundamentally quantum in nature, meaning that each wave is composed of indivisible packets of radiation called photons. As used in this context, the word “radiation” refers only to the fact that these waves travel. It does not necessarily indicate that these waves are harmful. Of the various frequencies of electromagnetic radiation, only extreme ultraviolet rays, x-rays, and gamma rays can directly cause mutation, cancer, and radiation sickness. These forms of high-energy radiation are known as ionizing radiation. See also: Cancer; Electromagnetic field; Electromagnetic wave; Electromagnetism; Energy; Gamma ray; Ionization; Light; Momentum; Mutation; Photon; Quantum mechanics; Radiation; Radiation injury to plants and animals; Ultraviolet radiation; Wave optics; X-ray

Types of electromagnetic radiation
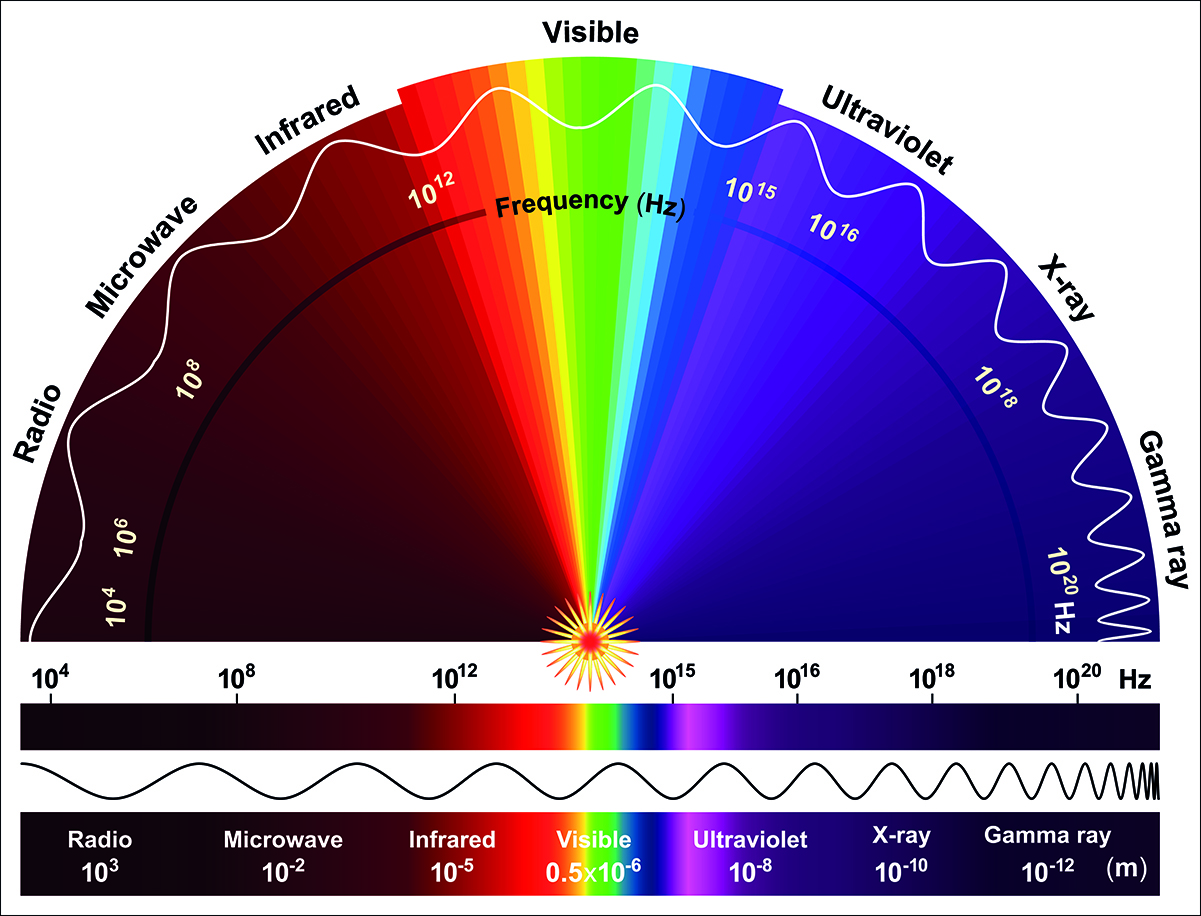
Electromagnetic waves exist across a wide range of frequencies and are therefore conventionally organized on a frequency spectrum (Fig. 2). Ordered from low to high frequency, the various types of electromagnetic radiation are: radio waves, infrared waves, visible light, ultraviolet rays, x-rays, and gamma rays. Radio waves have the lowest frequency and are commonly generated using antennas. Infrared waves are emitted by all objects through thermal radiation effects. Visible light waves include all colors visible to humans and are created through a wide variety of mechanisms. Ultraviolet rays, x-rays, and gamma rays are all on the high-frequency end of the spectrum. As such, they are only created in more exotic situations such as at extremely high temperatures or during nuclear reactions. See also: Antenna (electromagnetism); Color vision; Heat radiation; Infrared radiation; Nuclear reaction; Radio-wave propagation; Spectrum
Instead of being categorized by frequency, electromagnetic radiation can also be categorized according to the shape of its wavefront. Plane waves consist of radiation with planar wavefronts. While true plane waves cannot exist, many waves are approximately planar. An example is a typical laser beam. Spherical waves consist of radiation with spherical wavefronts, such as from a compact light bulb or radio antenna. Cylindrical waves consist of radiation with cylindrical wavefronts. See also: Geometrical optics; Laser; Wave motion
The emission of electromagnetic radiation
An electrically charged particle emits electromagnetic radiation any time it accelerates. There are several ways in which a charged particle can accelerate. These types of acceleration can be grouped into a few general categories.
Linear acceleration
This type of acceleration involves the particle abruptly gaining or losing speed. The most common case is when a high-speed particle enters a dense material, causing the particle to abruptly slow down.
- Braking radiation (bremsstrahlung) is emitted during the deceleration of moderately fast particles, such as occurs in traditional x-ray tubes. See also: Bremsstrahlung; X-ray tube
- Cerenkov radiation is emitted during the deceleration of extremely fast particles, such as occurs in the glowing water surrounding a nuclear reactor. See also: Cerenkov radiation; Nuclear reactor
Centripetal acceleration
This type of acceleration involves the particle traveling along a circular path.
- Cyclotron radiation is emitted by a particle circulating at relatively low speed, such as occurs in interstellar plasmas. See also: Cyclotron resonance experiments; Plasma (physics)
- Synchrotron radiation is emitted by a particle circulating at high speed, such as occurs in medical synchrotron machines. See also: Synchrotron radiation
Thermal motion
This type of motion involves the random accelerations of many charged particles. Thermal motion produces thermal radiation, which is temperature-dependent. See also: Temperature
- Infrared thermal radiation is continuously emitted by all objects in the universe. See also: Infrared radiation; Universe
- Incandescence is the emission of visible thermal radiation. Incandescence only occurs when objects are significantly hotter than room temperature, such as in wood fires, incandescent light bulbs, and the Sun. See also: Fire; Incandescence; Sun
Oscillatory motion
This type of motion involves the particle oscillating back and forth between two points in space or oscillating between two quantum states while making a transition.
- Antenna radiation is emitted when electrons oscillate inside an antenna, such as occurs in cell phones, radar guns, and radio broadcast towers. The simplest antennas emit a directional radiation pattern known as dipole radiation. See also: Electron; Mobile communications
- Radiation from a molecular transition is emitted when an entire molecule or a molecular electron transitions between quantized molecular states, such as occurs in carbon dioxide lasers. See also: Carbon dioxide; Molecule
- Radiation from an atomic transition is emitted when an electron transitions between quantized electron states in an atom, such as occurs in fluorescent light bulbs and neon signs. See also: Atom
- Radiation from a nuclear transition is emitted when an atomic nucleus transitions between quantum states, such as the gamma radiation emitted during radioactive decay. See also: Atomic nucleus; Radioactivity
- Radiation from an energy-band transition is emitted when an electron transitions between energy bands in a semiconductor, such as in a semiconductor laser or a light-emitting diode (LED). See also: Light-emitting diode
Particle-antiparticle annihilation
This process involves the complete annihilation of an electrically charged particle and its corresponding antimatter particle upon collision and the resulting production of two gamma ray photons. An example of this is in a patient undergoing a positron-emission tomography (PET) medical scan. See also: Antimatter; Medical imaging
The nature of electromagnetic radiation
All frequencies of electromagnetic radiation obey the laws of optics, which include refraction, reflection, dispersion, interference, and diffraction. Simple optical devices such as mirrors and lenses have been used since ancient times. In the early seventeenth century, the Dutch physicist Willebrord Snell (Fig. 3) empirically established the law of refraction, ushering in the age of modern optics. In the following decades, many advances in optics occurred around the globe. See also: Lens (optics); Mirror optics; Optics

During the nineteenth century, Scottish physicist James Clerk Maxwell organized all the laws of electricity and magnetism into one complete set, known as Maxwell’s equations. In the process, he found that these laws theoretically predict the existence of electromagnetic waves which obey the laws of optics. Within a few years, German physicist Heinrich Hertz experimentally confirmed Maxwell’s predictions using radio waves, thereby establishing the electromagnetic nature of light and unifying the fields of electromagnetism and optics. See also: Electricity; Magnetism
Electromagnetic radiation is fundamentally quantum in nature. This means that each wave consists of indivisible quantum packets called photons that obey the laws of quantum field theory. The most accurate description of electromagnetic radiation is currently quantum electrodynamics (QED), which was developed by a large number of scientists in the twentieth century. A major unsolved problem in physics is the determination of why electromagnetic radiation does not interact with dark matter, a mysterious substance theorized to constitute 85% of the matter in the universe. See also: Dark matter; Matter; Quantum electrodynamics





