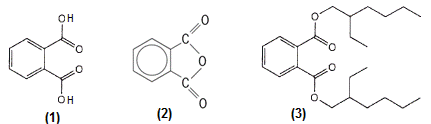
Phthalates are esters of 1,2-benzenedicarboxylic acid (phthalic acid) [1] made by reacting phthalic anhydride (2) with alcohols. Phthalates are used mostly as plasticizers—additives to make ridged polymers flexible. Flexible poly(vinyl chloride), or PVC (also known generically as vinyl), is the biggest application of these additives and can contain up to 30–40 percent of phthalates by weight. Some commonly used phthalates are di-2-ethylhexyl phthalate (DEHP) [3], also known as dioctyl phthalate (DOP); diisononyl phthalate (DINP); diisodecyl phthalate (DIDP); dipropylheptyl phthalate (DPHP); and ditridecyl phthalate (DTDP). See also: Acid anhydride; Carboxylic acid; Ester; Polymer; Polyvinyl resins
The application of flexible PVC is extensive. Some examples are vinyl flooring, cable and wire insulation and coatings, food processing equipment and packaging materials, medical devices such as tubing and intravenous bags, personal-care-product packaging, inks, shower curtains, and garden hoses. See also: Blood; Electrical insulation; Film (chemistry); Food manufacturing; Ink
Phthalates are controversial because they are suspected to be endocrine disruptors, chemical compounds that could interfere with the regulation of organisms’ hormone levels and which might have a particularly adverse effect on the reproductive systems of young, developing males. Some phthalates, such as DEHP, are considered potential carcinogens. The European Union (EU) has classified DEHP as a carcinogen, mutagen, reproductive toxin, and endocrine disruptor, and prohibits its use except for specifically approved applications. See also: Endocrine system (vertebrate); Environmental endocrine disruptors; Environmental toxicology; Mutagens and carcinogens
Because of the physical nature of phthalates and the sheer abundance of them (roughly three million metric tons) produced and used worldwide annually, at least small quantities of phthalates constantly permeate the environment: the air, the water, the soil, and the tissues of living things. At room temperature, phthalates are liquids that tend not to evaporate readily because they have characteristically high vapor pressures. Over time, however, these plasticizers may migrate to the surfaces of polymer products (of vinyl flooring, for example) and become airborne on dust particles or they may be extracted by fluids flowing through flexible PVC tubing (as in milk processing) or by leaching into foods wrapped in PVC packaging materials. Phthalates with longer and branched alcohol chains, such as DIDP, DPHP, and DTDP, are considered safer because they are less likely to migrate or be extracted. See also: Air pollution, indoor; Food; Vapor pressure; Water pollution
Finding suitable replacements for phthalates in PVC has not been easy because of the higher cost of alternative plasticizers or their less than satisfactory performance, such as poor kink resistance in medical tubing. The plasticizer n-butyryl tri-n-hexyl citrate (BTHC) has been found to be an acceptable replacement in PVC bags used to store blood and blood products because it exhibits low toxicity and has safe metabolites. Also finding acceptance as alternatives to flexible PVC are engineered polymers and copolymers called plastomers (polyolefin thermoplastic elastomers), which have built-in flexibility and do not require the use of plasticizers. Metallocene catalysts are used for the synthesis plastomers because they can precisely produce uniform polymers and copolymers with tailored structures and physical properties. See also: Catalysis; Copolymer; Metallocenes; Polymer stereochemistry and properties





