An exploding fireball is a difficult phenomenon to examine given its fleeting, rapidly evolving, and hazardous nature. A specific type of laser is now offering researchers a new way to study explosions, though, at safe distances and with the attendant sensitivity needed to capture sudden changes in temperature, pressure, and chemical concentrations. The hope is that probing explosions in this manner will aid in better controlling them for technological applications, as well as designing protective shielding from explosives. See also: Explosive; Explosive forming; Laser; Pressure; Temperature; Thermodynamic principles
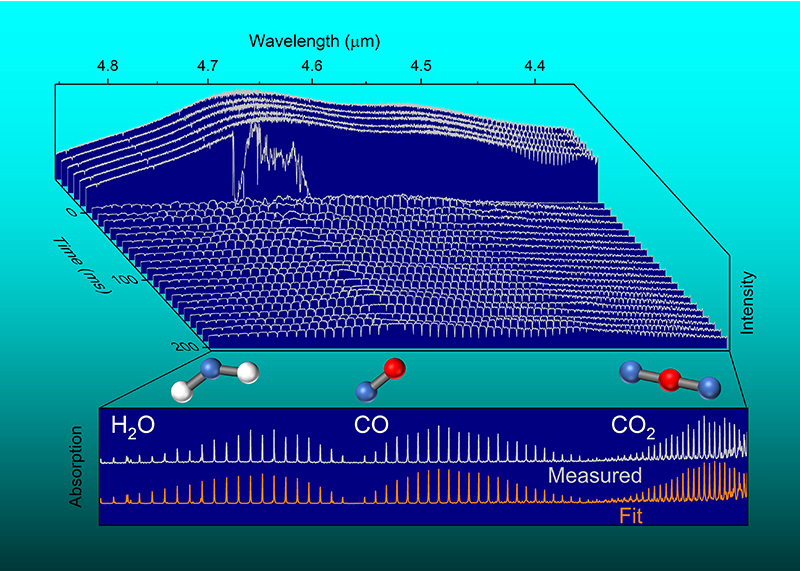
The laser in question is known as a swept-wavelength external-cavity quantum cascade laser (swept-ECQCL). When shone through a fireball, infrared light in the laser beam is absorbed by molecules including carbon dioxide, carbon monoxide, water vapor, and nitrous oxide. Each substance produced in the fireball absorbs the light in a distinctive pattern. Examining those signatures reveals the temperature and concentrations of those molecules, along with tiny particles of soot as the explosion unfolds, painting a complete picture of the event. See also: Absorption of electromagnetic radiation; Carbon dioxide; Infrared radiation; Light; Molecule; Water
The swept-ECQCL approach allows for real-time, comprehensive readings of explosions, in contrast to conventional techniques. These techniques have involved setting up robust temperature- or pressure-measuring devices to record data from inside a fireball; however, the measuring devices, while proficient at gathering their respective kinds of data, fail to gather information on chemical changes. Analysis of the leftover products from an explosion does offer important insights but likewise cannot collect moment-to-moment information on the explosive process itself. Yet room for refinement remains. Researchers plan to expand the range of frequencies available to swept-ECQCL, as well as boost resolution and scan rates, building upon the understanding of explosions as gained to date. See also: Infrared spectroscopy; Time





