Key Concepts
One of the two major classes of nucleic acids, mainly involved in translating the genetic information carried in deoxyribonucleic acid (DNA) into proteins. Ribonucleic acid (RNA) [Fig. 1] is an essential biological macromolecule (specifically, a nucleic acid) that is present in all living cells. The chief function of RNA is cellular protein synthesis. In other words, RNA converts the instructional genetic information stored in deoxyribonucleic acid (DNA) into proteins. It should be noted that some viruses use RNA (rather than DNA) to carry the genetic information. See also: Deoxyribonucleic acid (DNA); Genetics; Nucleic acid; Protein; Virus
Various types of RNAs (see table) function in protein synthesis. For example, transfer RNAs (tRNAs) and ribosomal RNAs (rRNAs) function in the synthesis of all proteins, whereas messenger RNAs (mRNAs) are a diverse set, with each member specifically directing the synthesis of one protein. Messenger RNA is the intermediate in the usual biological pathway of DNA → RNA → protein. However, RNA is a very versatile molecule. Other types of RNA serve other important functions for cells and viruses, such as the involvement of small nuclear RNAs (snRNAs) in mRNA splicing. In some cases, RNA performs functions typically considered DNA-like, such as serving as the genetic material for certain viruses, or roles typically carried out by proteins, such as RNA enzymes or ribozymes. See also: Enzyme; Ribozyme
| RNA type | Abbreviation | Cellular process | Function |
|---|---|---|---|
| Messenger RNA | mRNA | Translation | Template for protein synthesis |
| Ribosomal RNA | rRNA | Translation | Assembly of mRNA, tRNA, and translation factors for peptide bond formation |
| Transfer RNA | tRNA | Translation | Selection, activation, and transport of amino acids to the ribosome |
| Small nuclear RNA | snRNA | Pre-mRNA splicing (removal of introns and joining of flanking exons) | Spliceosome components: specification of sites of exon-intron cleavage (5′ and 3′ splice sites) and branch-point A residue (nucleophile) |
| Small nucleolar RNA | snoRNA | Pre-rRNA maturation | Specification of sites of modified nucleoside components (2′-O-methylnucleosides and pseudouridine) |
| Small Cajal body RNA | scaRNA | Maturation of snRNAs and snoRNAs | Specification of sites of modified nucleoside components (2′-O-methylnucleosides and pseudouridine) |
| Small RNA | sRNA | Pre-RNA maturation | Counterpart in Archaea of snoRNA in eukaryotes |
| Guide RNA | gRNA | mRNA editing | Small antisense RNA that provides the information for editing |
| MicroRNA | miRNA | RNA interference | Small, naturally occurring antisense RNA involved in gene regulation |
| Small interfering RNA | siRNA | RNA interference | Processed counterpart of miRNA, mediating inactivation or degradation of complementary mRNA or viral RNA |
| Transfer-messenger RNA | tmRNA | Translation | Rescues stalled ribosomes |
Structure and synthesis
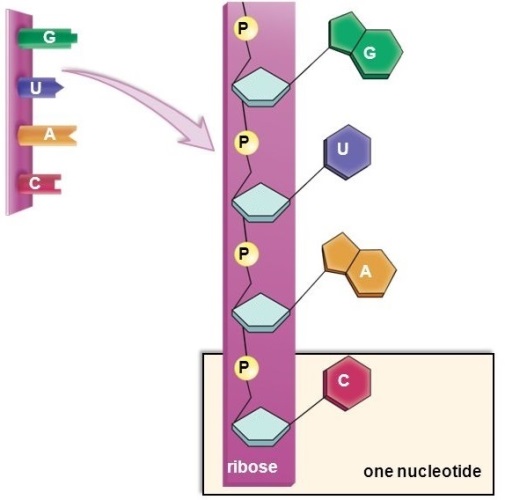
RNA is a linear polymer of four different nucleotides (Fig. 1). Each nucleotide is composed of three parts: a five-carbon sugar known as ribose; a phosphate group; and one of four bases attached to each ribose. The four bases are adenine (A), cytosine (C), guanine (G), and uracil (U). The combination of base and sugar constitutes a nucleoside. The structure of RNA is basically a repeating chain of ribose and phosphate moieties, with one of the four bases attached to each ribose. The structure and function of the RNA vary depending on its sequence and length. See also: Nucleotide
In its basic structure, RNA is quite similar to DNA. It differs by a single change in the sugar group (ribose instead of deoxyribose) and by the substitution of uracil for the base thymine (T). Typically, RNA does not exist as long double-stranded chains as does DNA, but rather as short single chains with higher-order structure due to base pairing and tertiary interactions within the RNA molecule. Within the cell, RNA usually exists in association with specific proteins in a ribonucleoprotein (RNP) complex.
The nucleotide sequence of RNA is encoded in genes in the DNA, and it is transcribed from the DNA by a complementary templating mechanism that is catalyzed by one of the RNA polymerase enzymes. In this templating scheme, the DNA base T specifies A in the RNA, A specifies U, C specifies G, and G specifies C.
Transfer RNA
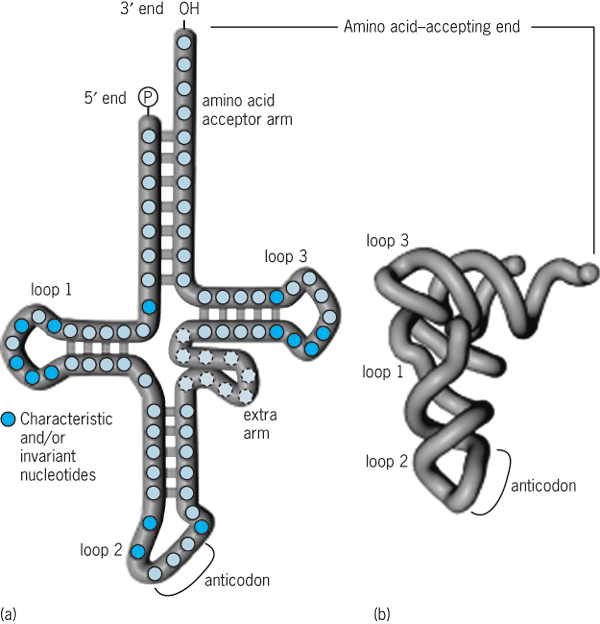
Transfer RNAs are small RNAs (70–90 nucleotides). They act as adapters that translate the nucleotide sequence of the mRNA into protein sequence. They accomplish this by carrying the appropriate amino acid to ribosomes during the process of protein synthesis. Each cell contains at least one type (usually several types) of tRNA specific for each of the 20 amino acids. The tRNAs exist in a precisely folded, three-dimensional, L-shaped structure (Fig. 2). Complementary base-pairing interactions between different parts of the tRNA play a major role in specifying this structure, with additional contributions from higher-order interactions. At one end of the L shape is the three-nucleotide anticodon sequence that binds its complementary sequence in the mRNA; at the other end is the amino acid specified by the mRNA codon. The base sequence in the mRNA directs the appropriate amino acid–carrying tRNAs to the ribosome to ensure that the correct protein sequence is made. Upward of 25% of tRNA nucleosides is unusual in containing modifications (such as methylations) on either the base or sugar component (or both). Among other roles, these modified bases are thought to facilitate recognition of the mRNA codon by tRNA. In the tRNA tertiary structure, a variable-length extra arm is accommodated within the hinge region that joins the two parts of the L-shaped structure. Few functions have been ascribed to the extra arm; however, in a few cases, it is known to contribute essential identity elements (in the form of its overall structure and/or specific nucleoside residues within it) for correct amino acid acceptor activity. See also: Amino acid
Ribosomal RNA
Ribosomes are complex ribonucleoprotein particles that act as workbenches for the process of protein synthesis, that is, the process of linking amino acids to form proteins. Each ribosome is made up of several structural rRNA molecules and more than 50 different proteins, and it is divided into two subunits, termed large and small. A bacterial ribosome contains one copy each of 5S rRNA (approximately 120 nucleotides) and 23S rRNA (approximately 2900 nucleotides) in its large subunit, and one copy of 16S rRNA (approximately 1540 nucleotides) in its small subunit. In eukaryotic (nucleus-containing) organisms, these rRNAs are also present in somewhat longer versions in ribosomes of greater size that also contain an additional rRNA of 5.8S (approximately 160 nucleotides) in their large subunit. See also: Ribosomes
The RNA components of the ribosome account for more than half of its weight. Similar to tRNAs, rRNAs are stable molecules and exist in complex folded structures. Each of these rRNAs is closely associated with ribosomal proteins and is essential in determining the exact structure of the ribosome. In addition, the rRNAs, rather than the ribosomal proteins, are likely the basic functional elements of the ribosome. They are actively involved at most, if not all, stages of protein synthesis. In this process, they make direct and sometimes transient contacts, via short stretches of base pairing, with the mRNA, tRNA, and each other.
The nucleotide sequences of many rRNAs from different organisms have been characterized. The secondary stem-loop structures adopted by the RNAs are more highly conserved than the overall primary sequence, indicating that the higher-order structure is the functional one. Because these RNAs are present and perform the same central function in all known organisms, they are well suited for defining evolutionary relations.
Messenger RNA
Whereas most types of RNA are the final products of genes, mRNA is an intermediate in information transfer. It carries information from DNA to the ribosome in a genetic code that the protein-synthesizing machinery translates into protein. Specifically, mRNA sequence is recognized in a sequential fashion as a series of nucleotide triplets by tRNAs via base pairing to the three-nucleotide anticodons in the tRNAs. There are specific triplet codons that specify the beginning and end of the protein-coding sequence. Thus, the function of mRNA involves the reading of its primary nucleotide sequence rather than the activity of its overall structure. Messenger RNAs are typically shorter-lived than the more stable structural RNAs, such as tRNA and rRNA. See also: Genetic code
In eukaryotes, mRNAs are derived from larger precursor RNAs by a series of RNA processing steps that take place within the cell nucleus. Additionally, the informational, or coding, sequences (exons) of most genes are interrupted by long stretches of noncoding sequences known as introns. A typical mRNA precursor consists of alternating exons and introns, with most of the RNA being introns. These long pre-mRNAs must be spliced to remove noncoding intervening sequences. The mRNA splicing process, which consists of RNA cleavage at junctions between exons and introns and ligation of exons, is mediated by another class of cellular RNAs—the small nuclear RNAs (snRNAs). See also: Exon; Gene; Intron
Small nuclear RNA
Small nuclear RNAs, generally less than 300 nucleotides in length and rich in uridine (U), are localized in the nucleoplasm (snRNAs) and nucleolus (snoRNAs) of eukaryotic cells, where they take part in RNA processing. These RNAs have characteristic folded structures and function as ribonucleoproteins (snRNPs and snoRNPs), in association with specific proteins. Several snRNPs participate in intron removal during eukaryotic mRNA splicing, whereas specific snoRNPs participate in the extensive posttranscriptional modification that occurs during production of mature rRNA. See also: Cell nucleus; Histone; Transcription
Antisense RNA (microRNAs and small interfering RNAs)
Antisense RNA is an RNA transcript (or portion of one) that is complementary to another nucleic acid, usually another RNA molecule. As mentioned previously, naturally occurring examples of antisense RNA include the snoRNAs that mediate site-specific cleavage and modification of rRNA precursors, and guide RNAs (gRNAs) that supply the information for the uridine insertion or deletion type of RNA editing. In both bacterial and eukaryotic cells, other naturally occurring antisense RNAs function to regulate gene expression by binding to a target RNA molecule and interfering with its normal function. In eukaryotic cells, introduction of antisense RNA (for example, through introduction and activation of an appropriate transgene) has been shown to modulate expression of the complementary gene. This observation has encouraged attempts to use antisense RNA to manipulate gene expression (gene silencing) for therapeutic and other purposes. See also: Gene silencing
An unexpected and exciting consequence of antisense RNA studies has been the discovery of small interfering RNAs (siRNAs) that mediate the phenomenon of RNA interference (RNAi). An enzyme called Dicer generates siRNA via endonucleolytic cleavage of long double-stranded RNA molecules, yielding siRNA products that are approximately 20–30 nucleotides in length. Small interfering RNAs that are complementary to a long RNA molecule are able to target that molecule for degradation, thereby affecting RNA stability. Introduction or expression of mRNA-specific siRNAs in target cells is a powerful means of selectively and comprehensively inactivating gene expression. See also: RNA interference; Small interfering RNA (siRNA)
MicroRNAs (miRNAs) are another class of small RNA species, and they constitute a natural counterpart of siRNAs. They are the same size as siRNAs and are also generated by Dicer, although miRNAs are processed from longer single-strand precursors that are capable of assuming a hairpin configuration. MicroRNAs specifically regulate the activity of target genes, usually through translational arrest as a result of complementary base pairing with a sequence within the 3′ untranslated region of the corresponding mRNA. They appear to be key components of a novel RNA-based system of gene regulation that is evolutionarily conserved among at least multicellular eukaryotes and that controls timing of development, stem cell maintenance, and other developmental and physiological processes in plants and animals. See also: MicroRNA
Viral RNA
Whereas most organisms carry their genetic information in the form of DNA, certain viruses, such as polio and influenza viruses, have RNA as their genetic material. The viral RNAs occur in different forms in different viruses. For example, some are single-stranded and some are double-stranded; some occur as a single RNA chromosome, whereas others are present as multiple different RNA species. In any case, the RNA is replicated as the genetic material and either its sequence or a complementary copy of itself serves as mRNA to encode viral proteins. The RNA viruses known as retroviruses contain an enzyme that promotes synthesis of complementary DNA in the host cell, thus reversing the typical flow of genetic information in biological systems. Many transforming viruses, which convert healthy cells to tumor cells, are retroviruses, as is human immunodeficiency virus (HIV) [the causative agent of acquired immune deficiency syndrome (AIDS)]. See also: Acquired immune deficiency syndrome (AIDS); Animal virus; Retrovirus
Catalytic RNA
RNA enzymes, or ribozymes, are able to catalyze specific cleavage or joining reactions either in themselves or in other molecules of nucleic acids. For example, one of the ribonucleases involved in tRNA processing, RNase P, is composed of one protein and one RNA molecule. Under certain conditions, the enzymatic activity can be supplied entirely by the RNA moiety. Several other types of catalytic RNA are categorized by specific conserved features of primary and secondary structures. The realization that RNA can function both as a catalyst (enzyme) and as an informational molecule has spawned the concept of a primordial "RNA World," with RNA preceding both DNA and protein in the evolution of the genetic information pathway. See also: Catalysis and catalysts; RNA World theory
Other types of RNA
RNA molecules serve other important and diverse cellular functions. For example, a ribonucleoprotein enzyme (telomerase) is responsible for replication of chromosome ends, with the RNA component functioning as a template for the reverse transcriptase activity of telomerase. An essential RNA component is also present in the signal recognition particle (SRP), a ribonucleoprotein complex that directs ribosome-synthesizing membrane and secretory proteins to the endoplasmic reticulum, where synthesis and posttranslational processing are completed. Transfer-messenger RNA (tmRNA) functions during translation to rescue stalled ribosomes, targeting aberrant, partially synthesized proteins for proteolytic degradation. Noncoding RNAs (ncRNAs), comprising transcripts representing essentially the entire eukaryotic genome, are implicated in transcriptional regulation, epigenetic gene regulation, and disease. As with eukaryotes, bacteria use a variety of RNA molecules to regulate gene expression, including CRISPR (clustered regularly interspersed short palindromic repeat) RNAs that inhibit the uptake of foreign DNA. See also: Chromosome; CRISPR/Cas9 gene editing; Endoplasmic reticulum
RNA editing
RNA editing describes several different posttranscriptional processes that alter the nucleotide sequence of a mature RNA species compared to that of the gene that encodes it. In the case of mRNA, RNA editing results in codon substitutions that change the final protein sequence and that may also create new translation initiation and termination signals. Noninformational RNAs (rRNA and tRNA) sometimes undergo editing, as do certain viral RNAs.
Several different types of RNA editing have been described, such as insertions and deletions of uridine residues in trypanosome mitochondria, cytidine to uridine changes in plant mitochondria and chloroplasts, and adenosine to inosine substitutions in animal nuclei. Editing ranges from a single cytidine deamination to generation of more than 50% of the final sequence by uridine addition. In most cases, neither the biochemistry of editing nor the determinants of its specificity have been firmly established. In trypanosome mitochondria, small guide RNAs (gRNAs) base-pair with the pre-mRNA in such a way as to determine the positions at which insertion or deletion of uridine residues occurs, thereby creating translatable open reading frames that did not exist prior to editing. Through approaches involving biochemical and genetic characterization, considerable progress has been made in identifying the RNA and protein components of editing complexes (editosomes) in several editing systems.





