Key Concepts
A source from which energy can be obtained to provide heat, light, or power. The term energy is used to describe an amount of work performed. There are two kinds of energy, kinetic energy, meaning work performed by the movement of matter, and potential energy, meaning work that is stored or at rest in matter. In the kinetic or potential state, energy takes on one of five forms: (1) Chemical energy results from changes in the chemical structure of substances, such as during combustion of fuel. (2) Electrical energy results from electrons and protons in motion in a stream called an electric current, or in temporary storage as in a battery or fuel cell. (3) Mechanical energy results from force applied or about to be applied to liquid, solid, or gaseous matter. (4) Thermal energy results from heat being applied to matter. (5) Nuclear fission is the splitting of the nucleus of an atom into two or more parts by collision with neutrons, with the consequent release of the force that binds protons and neutrons of the nucleus together. All living things on Earth depend on one or more of these forms of energy and must look to a wide variety of energy sources. See also: Battery; Chemical energy; Energy
Renewable energy sources are forms of potential energy that constantly and rapidly renew themselves for steady, reliable use. Solar and wind power are examples of renewable energy (Fig. 1). Any form of potential energy that does not fall within the definition accepted for renewable energy is considered nonrenewable. For example, the fossil fuels are nonrenewable energy sources (Fig. 2).

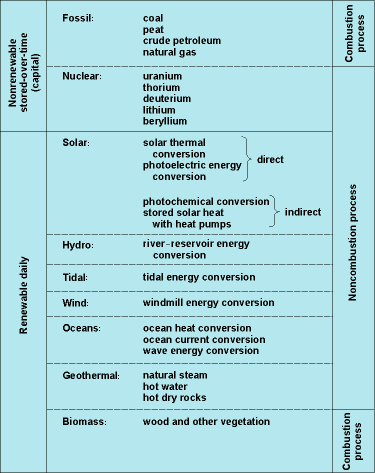
Fossil fuels
Crude petroleum, natural gas, and coal were formed in the Earth's crust over the course of millions of years and exist today in subsurface locations. Crude petroleum is found trapped in the pores of rock (sandstone, limestone, or dolomite) or sand overlain with some kind of impervious cap rock that prevents the liquid from dispersing. Natural gas is normally found trapped in the Earth alongside or with crude petroleum.
Coal
The principal chemical constituents of coal are water, carbon, hydrogen, sulfur, nitrogen, oxygen, and ash (noncombustible mineral residue). However, coal is not a uniform substance. It is almost infinitely variable in its composition from one location to the next, even within the same mine location. Different coals can be ranked according to the degree to which they have progressed from lignite through the bituminous stage to anthracite. This progression is roughly equivalent to the geologic time of development, although the other variables of depth-pressure, heat, and vegetable matter constituents play an important part. See also: Coal; Lignite
Coal is used for generating electric power, metallurgical production, general industrial processes, residential-commercial uses, and synthetic fuels. For the most part, coal is burned in fire-tube or water-tube boilers for raising steam that is used to generate electricity, provide heat for factories and buildings, and provide steam for production processes.
Peat
In the very early stages of coal formation, accumulations of decomposed and partially decomposed vegetation, trees, ferns, and mosses located in a wet, cold, and anaerobic (oxygen-deficient) environment are very likely to turn to peat at the rate of about 7.5 cm (3 in.) per 100 years. Only after hundreds or even millions of years would this material graduate to the status of coal.
Since the vegetable matter in peat, consisting of cellulose and other organic compounds, transforms only partially to carbon and hydrocarbons, peat has only one-third to one-half of the heating value of coals. It is used in very limited quantities for fuel after it is cut from the earth and formed into briquettes. See also: Peat
Crude petroleum
Oil is also a complex substance derived from the carbonized remains of trees, ferns, mosses, and other types of vegetable matter. The principal chemical constituents of oil are carbon, hydrogen, and sulfur. Crude petroleum extracted from the earth will burn and produce thermal energy, but virtually all crude oil is processed in refineries, where it is converted into several useful fuels and special products (for example, feedstock for chemicals, plastics, food products, medicines, and tires, plus tar, asphalts, and lubricating oils). The various fuels made from crude oil are jet fuel, gasoline, kerosene, diesel fuel (or No. 2 fuel oil), and heavy fuel oils (or No. 4, 5, and 6 fuel oils). Major oil consumption is linked to transportation, residential-commercial uses, industry boilers and other industrial uses, and for generating electric power. See also: Gasoline; Kerosene; Petroleum; Petroleum products
Natural gas
This energy source is 83–93% methane (CH4), so that its principal chemical constituents are carbon and hydrogen. Natural gas is usually found in the immediate vicinity of crude petroleum, although some natural gas wells do not yield oil.
Of all the chemical or mineral sources of energy, natural gas may well be the most desirable because it can be pipelined directly to the customer, requires no storage vessels, requires no air-pollution control equipment, produces no ash for disposal, and mixes with air easily to provide complete combustion at low excess air.
The principal uses for natural gas are residential, commercial, industrial, transportation-related, and for generating electric power. See also: Natural gas
Nuclear energy
The two types of nuclear energy are fission and fusion. In fission, heavy atoms are split into two principal elements that form the nucleus of two new, smaller atoms. In fusion, the nuclei of two small atoms fuse together into a single, larger nucleus. In both cases, large quantities of energy are released. See also: Nuclear power
Nuclear fission
Fission reactions involve the breakup of the nucleus of high-mass atoms. They yield an energy release which is more than a million times greater than that obtained from chemical reactions involving the burning of a fuel. The fission process involves the bombardment of atoms with neutrons so that a sufficient number of collisions will take place on a statistically predictable basis and split those atoms into two or more separate nuclei while releasing massive quantities of thermal energy. Nuclear power reactors produce steam for electric power production, for ship propulsion, or for process heat (Fig. 3). See also: Nuclear fission; Nuclear fuels

Nuclear fusion
The fusion process is the opposite of fission. Instead of splitting atoms into two or more pieces, the fusion process causes two atoms to collide with such force that their natural electronic repulsion is overcome and their nuclei fuse into one. Interest in the nuclear fusion reaction arises from the expectation that it may someday be used to produce useful power. Since a primary fusion fuel, deuterium, occurs naturally and is obtainable in virtually inexhaustible supply (by separation of heavy hydrogen from water, 1 atom of deuterium occurring per 6500 atoms of hydrogen), solution of the fusion power problem would permanently solve the problem of the present rapid depletion of chemically valuable fossil fuels. In power production the lack of radioactive waste products from the fusion reaction is another argument in its favor as opposed to the fission of uranium. Although this energy source offers tremendous potential for the twenty-first century, there are formidable problems that must be overcome before it can be utilized. Nevertheless, fusion power promises to be a source of continuous low-cost energy, provided that the technical problems can be solved. See also: Nuclear fusion
Solar energy
By far the most attractive energy source is the Sun itself because it is free, clean, and nonpolluting, and does not involve the use of dwindling, finite reserves of fossil fuels. Solar energy is used to provide heat for space comfort conditioning of buildings, for industrial processes, and for generating electricity. Photovoltaics convert solar energy directly into electricity. Solar energy can also be converted to useful work or heat by using a collector to absorb solar radiation, allowing much of the Sun's radiant energy to be converted to heat. This heat can be converted to electrical power. This is known as concentrating solar power (CSP). See also: Photovoltaic cell; Solar cell; Solar energy; Sun
Hydro energy
One of the oldest energy-producing mechanisms uses water flowing in a river or falling from a height to rotate work devices, ranging from the waterwheels of the past to the massive modern hydroelectric dams that employ gigantic electricity-generating turbines (Fig. 4). See also: Electric power generation; Waterpower

Tidal energy
The first major tidal energy project in operation was the Rance River project in Brittany, France. The French project employs a barrage type of dam across the estuary of a river. Turbines located in this barrage pump water into the estuary when the tide is rising. When a sufficient head of water is built up in the estuary, the water is permitted to flow back through the turbines to produce electricity. See also: Tidal power
Wind energy
Wind power is a renewable energy source that poses virtually no environmental problems (Fig. 1). However, wind power has limitations. Wind turbines can be located only where there is adequate wind. These high-wind areas may not be easily accessible or near existing high-voltage lines for transmitting the wind-generated energy. Another disadvantage occurs because the demand for electricity varies with time, and electricity production must follow the demand cycle. See also: Wind power
Ocean energy
Ocean power may become an energy source in the future, either as wave power or as ocean temperature differential. In Scandinavia, a wave-power device was designed for exploiting the wave motion of the sea, such that ocean waves cause massive quantities of water to flow into the confines of the device which then generates electricity as the water tries to escape back to the sea. An energy-producing system known as ocean thermal energy conversion (OTEC) is based on the temperature differential in the oceans near the Equator where the surface water is about 20°C (40°F) warmer than the water a few thousand feet down. This temperature difference can be utilized to vaporize a working fluid (such as ammonia) that can be run through an electricity-producing turbine.
Geothermal energy
Geothermal energy production is based thermodynamically on the temperature difference between a mass of subsurface rock and water and a mass of water or air at the Earth's surface. This temperature difference allows production of thermal energy that can be either used directly or converted to mechanical or electrical energy. The use of geothermal energy for electric power generation has become widespread because of several factors. Countries where geothermal resources are prevalent have desired to develop their own resources in contrast to importing fuel for power generation. Geothermal steam has become an attractive power generation alternative because of environmental benefits (Fig. 5). See also: Geothermal power

Biomass energy
As applied to the field of energy, the term biomass energy encompasses a broad selection of energy sources: Any and all types of living matter that can be converted to a form of energy can be said to be biomass, including wood, wood waste, coffee grounds, corn husks, peanut shells, rice hulls, garbage, animal and human waste, sugarcane waste (bagasse), and organic effluent from streams and ponds as biomass. For energy applications, biomass may be converted into liquid fuels or burned to generate heat or electricity. See also: Biomass





